 |
PIRSF Database
What is
PIRSF?
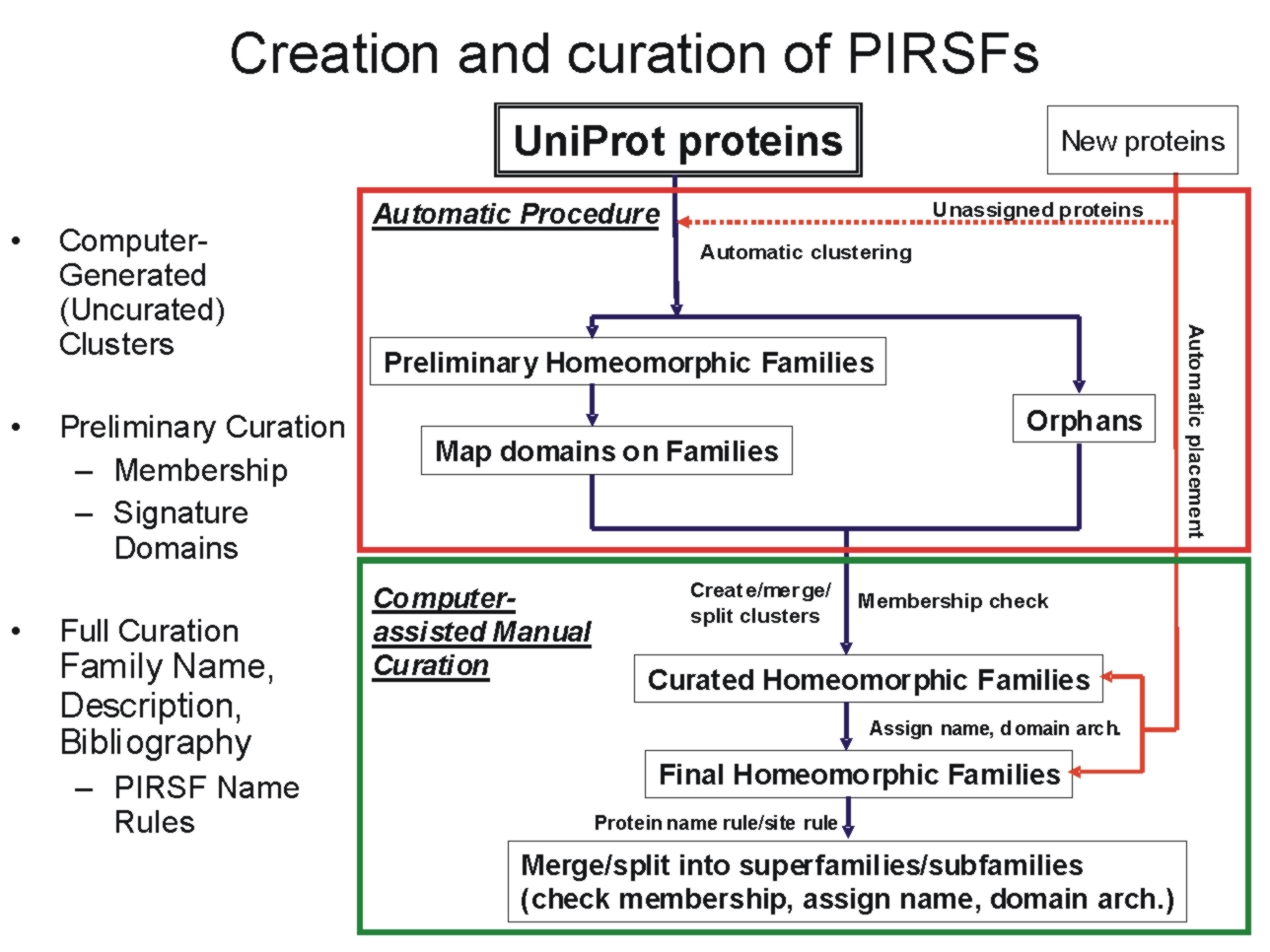
The PIRSF
protein classification
system is a network with multiple levels of
sequence diversity from superfamilies to
subfamilies that reflects the evolutionary
relationship of full-length proteins and domains.
The primary PIRSF classification unit is the
homeomorphic family, whose members are both
homologous (evolved from a common ancestor) and
homeomorphic (sharing full-length sequence
similarity and a common domain architecture).
Automatically generated protein clusters are
manually curated for membership, domain
architecture, annotation of sequence features, and
specific biological functions and biochemical
activities, when possible.
What in PIRSF for me?
PIRSF offers curated protein families with rules for functional site and protein name propagation and standardization, therefore, improving the sensitivity of protein identification and functional inference. Searching your protein sequence
against PIRSF database provides a faster and more accurate assessment of its function than a BLAST search against an
uncurated protein database. It avoids pitfalls such as numerous erroneous annotations, best hits based on a domain
secondary to the main protein function, spurious hits, etc.
PIRSF Definitions
-Curation Status
Uncurated: Computer-generated protein clusters, no manual curation. The clusters are computationally defined using both pairwise based parameters (% sequence identity, sequence length ratio and overlap length ratio) and cluster-based parameters (% matched members, distance to neighboring clusters and overall domain arrangement).
Preliminary: Computer-generated clusters are manually curated for membership (do proteins belong to the assigned cluster?) and domain architecture (Pfam domains listed from N- to C- termini).
Full/Full (with description): A name is assigned to the protein family, and accompanying references are listed when available. In many cases, brief descriptions are also provided.
-PIRSF MembershipFull (F): proteins
sharing end-to-end sequence similarity and common domain architecture.
Associate (A): members whose lengths are outside the family length range, including sequences fragments, alternate splice and alternate initiator variants, and peptides derived from
proteolytic processing, are classified as associate members with the conceptual complete sequence from which they are derived. Associate members also include individual proteins with atypical domain architecture (thus, not yet forming a separate subfamily).
Seed (S): full members that are used to generate family specific full-length and domain HMMs.
Use the following formats to perform a search in iProClass using PIRSF Membership in the search field: PIRSFxxxxxx:F, PIRSFxxxxxx:S or PIRSFxxxxxx:A for Full, Seed or Associate, respectively, with xxxxxx being the PIRSF number.
-PIRSF Name Evidence Tag
[Validated]: to indicate that at least one
member in the family has experimentally-validated function.
[Predicted]: for families whose functions
are inferred computationally based on sequence similarity and/or functional associative
analysis.
[Tentative]: cases where experimental evidence is not decisive.
-PIRSF Family Level
Homeomorphic family (HFam): members belonging to the family are both homologous (evolved from a common ancestor) and homeomorphic (sharing full-length sequence similarity and a common domain architecture). The homeomorphic family level is
the primary PIRSF curation level – and most significant in terms of annotation and most
invested with the biological meaning. A protein may be assigned to one and only one
homeomorphic family, which may have zero or more parent nodes and zero or more child
nodes.Homeomorphic families are assigned numbers < 500000, e.g., PIRSF001830
Subfamily (SubFam): The subfamily level is used to delineate protein clusters within a
homeomorphic family that have specialized functions and/or variable domain
architectures. Like its parent, each subfamily is also homologous and homeomorphic. A
protein may be assigned to zero or one subfamily, which will have exactly one parent
node. Subfamilies are assigned numbers ≥ 500000 and < 800000, e.g., PIRSF500000.
Superfamily (SuperFam): The superfamily level is used to bring together a number of distantly
related families and orphan proteins that share one or more domains. Depending on the
extent of domain coverage, a superfamily may be a “homeomorphic superfamily”
(common domain architecture with full-length sequence coverage) or a “domain
superfamily” (partial sequence coverage). Superfamilies are assigned numbers ≥ 800000, e.g., PIRSF800000.
-Domain Architecture
Pfam domains assigned with high confidence, either manually or automatically (for single domain-containing families), listed from N- to C- termini. PFam domains are separated by semi-colons, although in a few cases, domains are separated by a dash indicating the presence of inserted domains.
Numbers in parenthesis indicate the repetition of a domain. There is a particular syntax for this feature. For example, PF11111(1-3) allows for 1 to 3 copies of PF11111, whereas PF11111(2-) allows for any number of domains above 1 (2 or more).However, PF11111(0,2) allows for none or two copies of this domain.
-Representative Sequence
It is the sequence of a member of a PIRSF that belongs to a model organism (if present), belongs to UniProtKB/Swiss-Prot (if any) and meets the PIRSF criteria of uniform length and common domain architecture. Representative sequences are assigned automatically, but curator may decide to change it.
iProClass Database
What is iProClass?
iProClass provides summary descriptions of protein
family, function and structure for UniProt sequences, with links to over 90 biological databases
(See data sources).
iProClass comprises reports for all
UniProtKB
proteins and those proteins that are exclusively in UniParc database.
iProClass
Text Search
Retrieve a matching list of
summary reports by text string or unique
identifier (selecting a field from the
dropdown menu). Click "Search" button to retrieve
results. You may open extra input boxes in your
query by clicking on "Add input box". See Text
Search Help for additional
information.
iProLINK Help
What is iProLINK?
As PIR focuses its effort on the curation of the UniProtKB protein sequence database, the goal of iProLINK is to provide curated data sources that can be utilized for text mining research in the areas of bibliography mapping, annotation extraction, protein named entity recognition, and protein ontology development.
The process of applying literature mining methods for protein database curation involves several tasks:
- Bibliography Mapping: identification of articles from literature sources (such as PubMed) that describe a given protein entry;
- Annotation Extraction: categorization of annotation types and extraction of sentences and/or phrases describing the given annotation; and
- Database Curation: conversion of the extracted literature information into annotation in the database with structured syntax, controlled vocabulary, and evidence attribution.
|
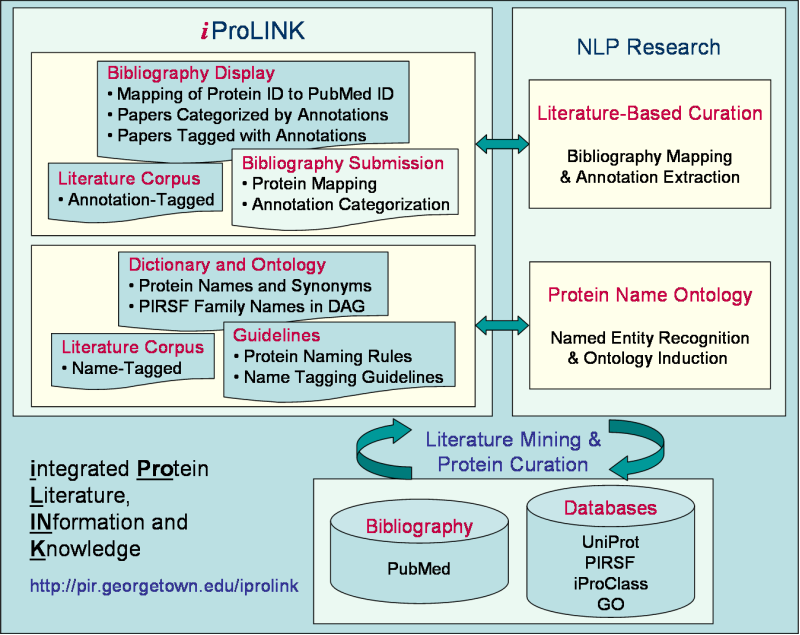 |
These tasks are also related to the topics of protein named entity recognition and protein ontology development.
A prerequisite to bibliography mapping is protein named entity recognition/identification of protein names from articles.
Furthermore, due to the long-standing problem of protein nomenclature, a protein ontology can assist entity recognition with the description of names and synonyms of protein classes as well as their relationships.
Bibliography Mapping
Linking protein entries to relevant scientific literature that describes or characterizes the proteins is crucial for increasing
the amount of experimentally verified data and for improving the quality of protein annotation.
Enter a text string or a unique identifier to retrieve bibliography related to you query.
Results are shown in a table similar to iProClass result page, but with specific columns related to bibliography retrieval. Links to the bibliography record as well as the individual PubMed entries are available.
iProLINK Bibliography Mapping Result Page for UniProtKB P13866

Feature Evidence Attribution
In the PIR-PSD database, feature annotation such as binding sites, catalytic sites, and modified sites are labeled with status tags "experimental" or "predicted" to distinguish experimentally verified from computationally predicted data. To appropriately attribute bibliographic data to features with experimental evidence,
a retrospective literature survey was conducted, which involves both citation mapping (finding citations from the Reference section that describe the given experimental feature) and evidence tagging (tagging the sentences providing experimental evidence in an abstract and/or full-text article). Now this is being extended to UniProtKB proteins.
Search for proteins with a particular feature attribution such as post- translational modifications, or enter a UniProtKB identifier to retrieve feature attribution for your query.
Later on you may get curated bibliography related to the entry by clicking on "Bibliography" or all available tagged evidence by clicking on "Tagged Evidence"
Examples for Feature Evidence Attribution Text Search
| Feature |
Example |
| Active site | ser |
| Binding site | heme |
| Cleavage site | arg |
| Cross-link | cys |
| Disulfide bonds | all |
| Modified site | block* |
| Domain | signal sequence |
| Product | dermorphin |
| Region | ATP binding |
iProLINK Feature Mapping Result Page for heme binding site

RLIMS-P
The RLIMS-P is a rule-based text-mining program specifically designed to extract protein phosphorylation information on protein kinase, substrate and phosphorylation sites from the abstracts (Hu et al., 2005). Submission of PMIDs as input, returns a summary table for all PMIDs with links to full reports . The summary table lists the PMID of each phosphorylation-related abstract along with its top-ranking annotation result, followed by a list of remaining PMIDs for abstracts containing no phosphorylation information. Full reports can be retrieved from the summary table using hypertext links (from “text evidence") or by selecting one or more PMID(s) in the list.
Summary Table for PMIDs 2108025, 16436437, 15193450
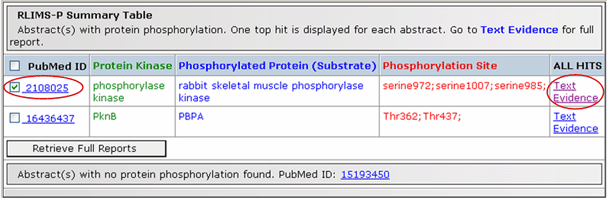
The full RLIMS-P report contains five sections (see table below): 1- PubMed citation information (publication date, authors, journal); 2- PMID mapping to UniProtKB, consisting of the accession, ID, protein name, organism, and protein family of the mapped entry, with links to UniProtKB and iProClass (Wu et al., 2004) protein reports containing rich biological and functional information; 3- Name mapping to UniProtKB, including options to use either names appeared in the abstract or user-specified names for searching online BioThesaurus; 4- Annotation with a ranked list of RLIMS-P extraction results for each set of the three phosphorylation objects; and 5- Text evidence showing the original abstract and title, with extracted objects tagged in different colors to distinguish protein kinases, phosphorylated proteins, and phosphorylated residues/positions. An option is provided for turning on/off the color-tagging for each type of object in the abstract for visual inspection.
Full Report for PMID 2108025

Entity Recognition/Ontology Development
Protein named entity recognition (finding protein names from literature texts) is a prerequisite for bibliography mapping (identifying papers describing specified proteins). It is also fundamental for several other biological literature mining tasks, including the extraction of protein annotations (such as protein-protein interactions) from literature.
-BioThesaurus
BioThesaurus is a web-based system designed to map a comprehensive collection of protein and gene names to UniProtKB protein entries. Currently covering more than two million proteins, BioThesaurus consists of over 2.6 million names extracted from multiple online resources based on database cross-references in iProClass. It allows the retrieval of synonymous names of given protein entries and the identification of ambiguous names shared by multiple proteins.
You can search by typing a gene/protein name, or alternatively, by using an identifier.
BioThesaurus Report for UniProtKB P18688

-Protein Name Dictionary and Word Token Dictionaries
PIR protein name dictionary is derived from the protein name field in the iProClass database, which consists of protein names from UniProt (Swiss-Prot,TrEMBL, PIR-PSD) and RefSeq. After the initial compilation, the dictionary undergoes several filtering processes to generate unique protein names (including synonyms and acronyms), and to remove nonsensical names and certain general descriptional annotations. For example, entry names such as "Inter-alpha-trypsin inhibitor (GIK-14) (Fragment)" were broken into Inter-alpha-trypsin inhibitor, GIK-14 and Fragment. The name Fragment is later removed from the dictionary along with a list of other "bad" names such as hypothetical protein, conserved hypothetical protein, unnamed protein product, predicted protein, and predicted protein of unknown function. In addition, words such as probable, putative, and similar to before protein names are also removed so that a name like putative aspartate aminotransferase A is merged to aspartate aminotransferase A to reduce the redundancy. Derived from over 1.5 million iProClass entries, the protein name dictionary currently has about 700,000 names, each of which is shown with its frequency count.
Most protein names are composed of combinations of two or more words (or tokens). Therefore, protein name rules can be derived from tokenized protein words and used during post-tagging processing to improve machine learning-based named entity recognition. We have compiled specialized single-word dictionaries by tokenization and classification of protein names from 30,000 well-curated iProClass protein entries (each containing at least 5 reference citations). The dictionaries consist of individual word tokens categorized into five classes.
- Biomedical Terms (bt): These terms are used in a broad of biological and medical sciences. They mainly describe structures of all forms of life at different levels (from gross morphology to molecular structure), as well as their respective functions and mechanisms in both normal (physiological) and diseased states (pathological).
- Chemical Terms (ct): These are words that describe organic or inorganic chemical materials, chemical groups or bonds, or chemical properties.
- Macromolecules (mc): These words refer to biopolymers such as proteins, peptides, DNA, RNA, polysaccharides, or glycoproteins.
- Common English (ce): Common English words are used to describe various aspects or properties of proteins, such as short, signal, interacting, and repair. These also include spelled-out forms of Greek letters, as well as stop words like of, at, and to.
- Non-word tokens: They are combinations of letters, numbers, or symbols. They often are acronyms, synonyms, or abbreviations. The form of non-word tokens can be number only, single letter, multiple letters, or combinations of numbers, letters, and other symbols. Non-word tokens may stand for biochemical entities such as nucleic acids, nucleotide, and amino acids, which can be used in a protein name.
-Protein Name Tagging Guidelines and Name-Tagged Corpora
Other iProLINK data resources for named entity recognition are two sets of literature corpora that were manually tagged with protein names based on two versions of tagging guidelines. Guideline 1.0 defines how to tag protein objects, not protein named entities, whereas Guideline 2.0 defines tagging rules for protein named entities regardless of the context of the object.
-PIRSF Family Classification-Based Protein Ontology
Biological ontologies are crucial for biological knowledge management, including mining literature data to extract relevant information and integrating information from multiple databases. A protein ontology?consisting of names and synonyms of protein classes as well as their relationships?can be used to assist with protein named entity recognition. Furthermore, an ontology based on protein family relationships, such as the PIRSF classification system, can be mapped to and complement the Gene Ontology (GO).
We have developed a protein ontology based on PIRSF hierarchical family names. The ontology is in the GO flat file format with a DAG (directed acyclic graph) structure, and can be browsed using application tools such as DAG-Edit.
Text Search Help
Select a
Database
Depending on the route by which
the Text Search page was accessed, you may need to
select between the iProClass database, which includes UniProtKB and unique UniParc proteins, and PIRSF database, which includes the whole set of PIRSF
families (i.e., any curation level). However, Text Search in the home page and the quick search box uses iProClass database.
The output file will display a table with individual protein entries or protein families, for iProClass or PIRSF, respectively.
Selecting a
Field
Searchable
fields are selected from the dropdown menu.
The items will vary according to database
selected, since each database contains different
types of information.
Query Input
Enter a unique
identifier or other search string in the box
provided.
Certain items (such as "Length" or
anything with "ID") will be exact match searches.
Other items will be substring searches (as if
preceded and followed by wild cards).
When the
field option is present, entering "not null" in
the text box will cause the search to return only
those entries that have some data in the selected
field, while entering "null" will return only
those that lack data in the selected field.
For
peptide search, type in a string of amino acid
residues in single
letter code (at least three letters), then
press the arrow to retrieve results.
Add Input Box Button
If
desired, multiple fields can be searched
simultaneously. Pressing the "+ box" button adds
another query line, up to a maximum of 8 lines.
The added input boxes are connected by logical
operator choices (see below), with the default
being the Add operator.
Using the Logical Operators AND,
OR, NOT
Search supports the logical 'AND',
'OR', and 'NOT' operators. For example, to
retrieve results that include Pfam domains A or B,
type A in your first query field and add a query
line by clicking the "Add input box" button. Enter
your 2nd query (B) and select the OR operator.
Similarly, to retrieve multi-domain proteins that
have both Pfam domains A and B, use the 'AND'
operator. Proteins that have domain A and not
domain B can be retrieved using the 'NOT'
operator.
Number of
Results
To provide the fastest
result, the default number of entries shown
on any one page is 50.
Search
Categorizations and Unique Identifiers
The
following table indicates the searchable fields
for Text and Batch Retrieval search
functions. Searchable fields can be selected from
the dropdown menu. You can search the databases
using unique identifiers or keywords. Entry
examples are shown below.
Text Search Result Help
This section describes the Text Search Result page for iProClass and PIRSF. The overall layout of the page is similar for both, however, it differs in the default columns that are displayed, as well as the tools available to analyze data.
Common features are described first 1-3, where numbers indicate the place where you will find these features in the page (see iProClass and PIRSF result pages below).
1- Search
This feature allows you to perform an additional search in case you want to further filter out your output
or you want to start a new search (no need to go back to the previous page unless you want to use a different database).
2- Display Options
Depending on your specific need you can
choose the columns to be displayed. To do this, click on the "Display Option" button, select the
relevant field(s) in the "Fields Not in Display"
list and transfer them to the "Fields in
Display" list via the ">" button. Conversely,
columns can be removed from display. Finally, click on "apply"
for the changes to take effect.
3- Save Results
As
The output can be saved to the
user's local computer. The results will be saved
for selected entries or, if no proteins are
selected, for all entries. Clicking "Table" will
save the displayed columns as a tab-delimited text
file, which may be imported into a spreadsheet for
easier viewing or analysis. Clicking "FASTA" will
save the IDs and sequences in FASTA
format.
iProClass Result Page
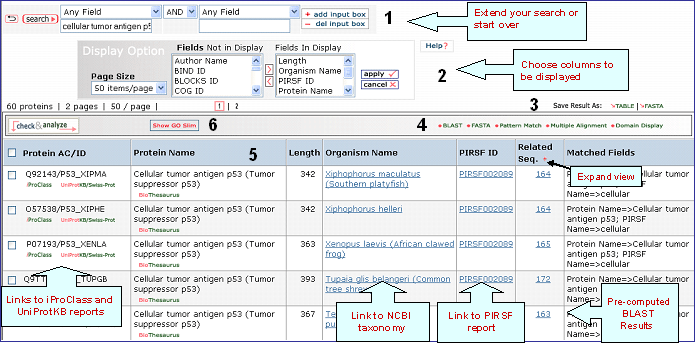
4- Analyze: BLAST,
FASTA, Pattern Match, Multiple Alignment and
Domain Display
Retrieved entries can be
further analyzed using the sequence analysis
programs available in the Results page. First,
select the protein(s) using the checkboxes on the
left side of the table, then click the
corresponding analysis tool. Click "BLAST" or
"FASTA" button, and a new query page will be
displayed, along with the parameters that were
selected in the initial search. Click "Pattern
Match" to search against the PROSITE database. For
multiple alignment, check at least 2 proteins (but
no more than 70), then click the "Multiple
Alignment" button. This will open the Multiple alignment form from which you can select one of the following alignment programs: ClustalW, T-Coffee or Muscle. The result page will display the alignment and alignment viewer. For ClustalW and T-Coffee, the neighbor-joining tree and alignment can be viewed, edited and saved using either PIR-TAV viewer or JalView. For Muscle only Jalview is available.
Domain display option, shows PFam domains (if
present) in graphical format.
5- Results Display
Results of
the search are displayed in a customizable table.
The exact columns displayed will depend on the fields searched for, and
user preference.
Protein AC/ID
The
Protein AC/ID refers to the UniProtKB or UniParc identifiers. Below these numbers, you may choose either the iProClass or the UniProtKB/UniParc view of the protein report. The source of the UniProtKB sequence is shown as UniProtKB/Swiss-Prot or UniProtKB/TrEMBL if the protein sequence is from Swiss-Prot or TrEMBL section, respectively. Alternatively, the UniParc ID will be displayed if the sequence is no present in the UniProtKB database along with the UniParc report.
Protein Name
The common
name given to a protein, that identifies its
function or specifies its features.
Length
Number of amino acid
residues in the peptide or protein.
Organism Name
The genus and species of the source organism from which the sequence originated. Links to NCBI taxonomy information are provided.
PIRSF ID
If a protein belongs to a PIRSF family, then this column will display the corresponding family identifier. Click on the ID to retrieve the PIRSF report (see annotated output).
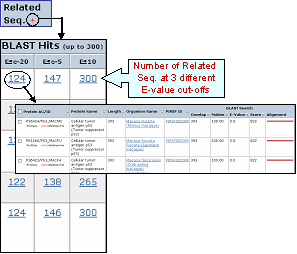
Related Seq.
This column shows the number of pre-computed BLAST hits obtained using default parameters. Only up to 300 sequences will be displayed. By clicking the number you access to the related sequence page. This allows to have a glance at sequence similarity in a very fast way. The number itself already provides some information about how unique the protein is. For example, a very low number may tell you that the query is specific to a certain species, genus, taxon, etc. The "+" sign next to the Related Sequence title allows to compare number of related sequences at 3 different E-value cut-offs as shown below.
Matched
Fields
The field(s) matched by the
query.
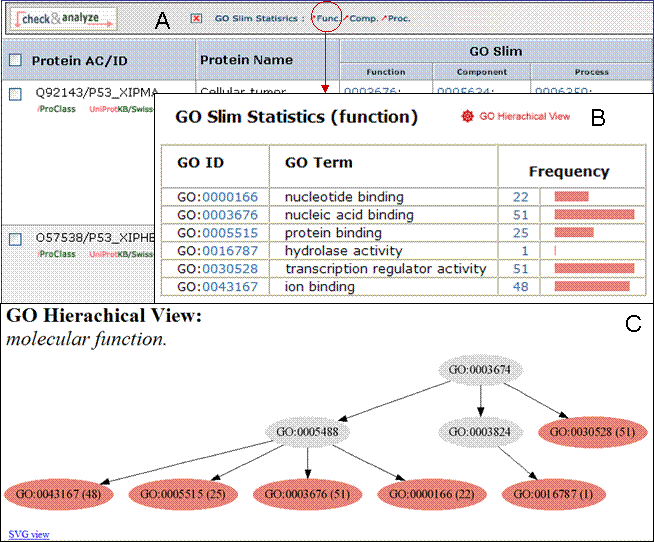
6- GO Slim
GO slims are smaller versions of the Gene Ontologies containing a subset of the terms in the whole GO. They give a broad overview of the ontology content without the detail of the specific fine grained terms. GO slims are particularly useful for giving a summary of the results of GO annotation of a genome or proteome when broad classification of gene product function is required.
You can view the GO slim terms for Biological Function, Component, and Process by selecting the “Show GO Slim” button in the analysis tool bar A. You can then view statistics for the individual ontologies (Function, Component, and Function) by checking entries of interest and selecting the ontology to show (for example, function in this example, A). Follow the GO ID links to learn more about the GO term. In addition, you can view the terms within the ontology by selecting the GO graphical hierarchical view icon (B). The graphical display will show the GO hierarchy in relation to the terms shown in the table C). Terms that map to a given protein are shown in color, the number in parenthesis indicates the number of proteins in the selected set that are annotated with the given term. Clicking on the number will retrieve the corresponding protein entries. An option to display the graph in svg format is provided. This format allows to rescale the image.
PIRSF Result Page
4- Analyze: Multiple Alignment, Taxonomic Distribution and
Domain Display
Retrieved entries can be
further analyzed using the sequence analysis
programs available in the Results page. First,
select the PIRSF(s) using the checkboxes on the
left side of the table, then click the
corresponding analysis tool. Click on the "Multiple
Alignment" button to generate an alignment
and neighbor-joining tree for selected PIRSFs. If only one PIRSF is selected, then alignment can be performed using the seed members or the full set of sequences (see below). Alternatively, you can modify the list, adding/deleting members. Please note that there is a maximum of 70 sequences for the alignment.
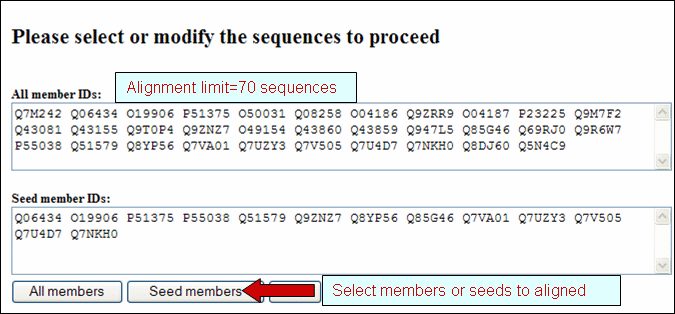
If more than one PIRSF is selected, a pop-up message will indicate that the representative sequences of each family will be used for the alignment.
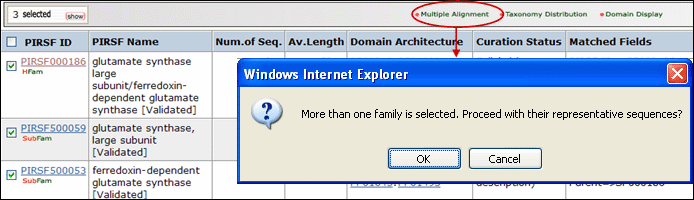
See sample output for selected PIRSF000186 members.
Click on Taxonomic Distribution to look at the number of members of a PIRSF present in the different taxa. You can perform this function on one or multiple PIRSFs. If only one PIRSF is selected, the taxonomic distribution for the parent family and the children (if any) will be displayed. See sample output for PIRSF000186.
Domain display option, shows PFam domains (if
present) in a graphical format.
5- Results Display
The results of
the search are displayed in a customizable table.
The exact columns displayed will depend on the fields searched for, and
user preference.
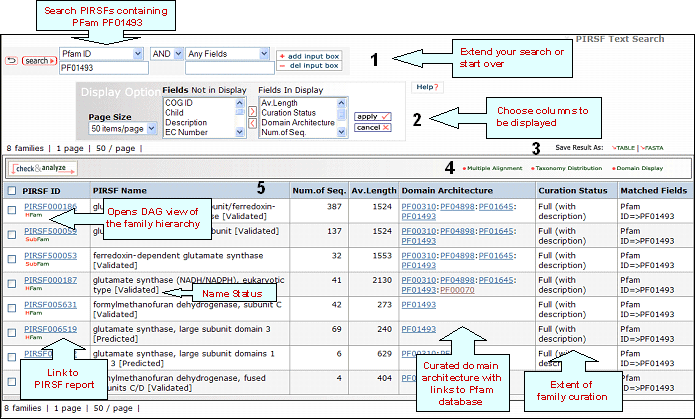
PIRSF ID
This column will display the corresponding family identifier. Click on the ID to retrieve the PIRSF report. The icon below the PIRSF ID states the family level, namely homeomorphic family (HFam), subfamily (SubFam), and superfamily (SuperFam). Clicking on this icon will bring the DAG view of PIRSF Hierarchy with the PFam domain at the higher level.
PIRSF Name
PIRSF name is meaningful for fully curated families since these names have been analyzed by a curator. The PIRSF Name aims at describing some common characteristic or function of the members in the family. Whenever possible PIRSF names are based on literature and try to follow any published standards. In most cases, names have an evidence tag or name status attached in brackets, which provides the level of confidence on the assigned name. The possible PIRSF name status tags are: validated (there is experimental data supporting PIRSF name), tentative (there is no conclusive experimental evidence) or predicted (predicted by computational methods).
Num. of Seq.
Number of sequences in the family.
Av.Length
It is the protein average length of the members of the PIRSF.
Domain Architecture
It represents the curated information regarding present Pfam domains in the protein family. Pfam domains are listed in order from N- to C- terminus separated by semi-colons. In a few cases, domains are separated by a dash indicating the presence of inserted domains.
Numbers in parenthesis indicate the repetition of a domain. There is a particular syntax for this feature. For example, PF11111(1-3) allows for 1 to 3 copies of PF11111, whereas PF11111(2-) allows for any number of domains above 1 (2 or more). However, PF11111(0,2) allows for none or two copies of this domain.
Curation Status
Uncurated: Computer-generated
protein clusters, not
curated.
Preliminary: Membership and
domain architecture of protein families determined
by manual curation.
Full:Protein family
name with accompanying references (when
available), and sometimes brief descriptions,
provided after thorough manual curation.
Matched
Fields
The field(s) matched by the
query.
Batch Retrieval
Help
Retrieve multiple entries
by selecting a specific identifier or a combination of identifiers.
Select the database
Before entering the protein identifiers you need to select the most convenient database. If you want to retrieve information about protein families, then select the PIRSF database, however if you are interested in analyzing individual proteins, then select the iProClass database.
Rules for entering
IDs
Multiple Entry
IDs should be separated by lines or
spaces.
IDs may be specified as a single
category or as mixed categories. However, if your entries have the same type of ID, it is recommended that you define the ID field to speed up the retrieval process.
You can further analyze and save any
retrieved results and/or their respective
sequences in a similar way as you do after a Text Search. Click on "Show Match List" to check the correspondence between your entry identifiers and the ones from the selected database.
Batch Retrieval Output in iProClass Database
Retrieval of sequences with GI numbers 1169968, 1707983 and 304131
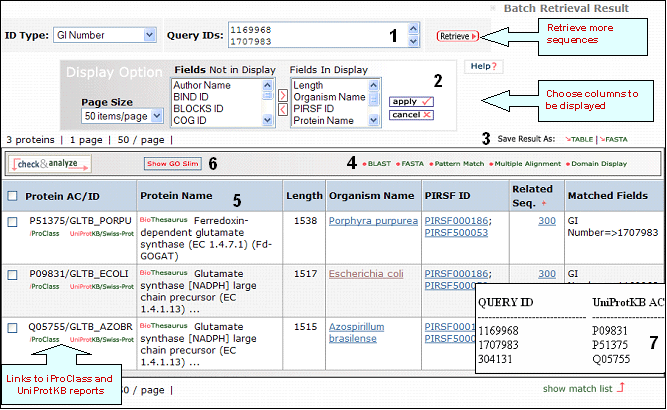
1- Retrieval Box
This box shows your query ID and also allows you to perform a new retrieval.
2- Display Options
Depending on your specific need you can
choose the columns to be displayed. To do this, click on the "Display Option" button, select the
relevant field(s) in the "Fields Not in Display"
list and transfer them to the "Fields in
Display" list via the ">" button. Conversely,
columns can be removed from display. Finally, click on "apply"
for the changes to take effect.
3- Save Results
As
The output can be saved to the
user's local computer. The results will be saved
for selected entries or, if no proteins are
selected, for all entries. Clicking "Table" will
save the displayed columns as a tab-delimited text
file, which may be imported into a spreadsheet for
easier viewing or analysis. Clicking "FASTA" will
save the IDs and sequences in FASTA
format.
4- Analyze: BLAST,
FASTA, Pattern Match, Multiple Alignment and
Domain Display
Retrieved entries can be
further analyzed using the sequence analysis
programs available in the Results page. First,
select the protein(s) using the checkboxes on the
left side of the table, then click the
corresponding analysis tool. Click "BLAST" or
"FASTA" button, and a new query page will be
displayed, along with the parameters that were
selected in the initial search. Click "Pattern
Match" to search against the PROSITE database or against a user defined pattern. For
multiple alignment, check at least 2 proteins (but
no more than 70), then click the "Multiple
Alignment" button. This will open the Multiple alignment form from which you can select one of the alignment programs: ClustalW, T-Coffee or Muscle. The result page will display the alignment and alignment viewer. For ClustalW and T-Coffee, the neighbor-joining tree and alignment can be viewed, edited and saved using either PIR-TAV viewer or JalView. For Muscle only Jalview is available.
Domain display option, shows PFam domains (if
present) in graphical format.
5- Results Display
Results of
the search are displayed in a table.
Protein AC/ID
The
Protein AC/ID refers to the UniProtKB accession number and id, respectively. Below these, you may choose either the iProClass or the UniProtKB/UniParc view of each protein report. The source of the UniProtKB sequence is shown as UniProtKB/SP or UniProtKB/Tr if the protein sequence is from Swiss-Prot or TrEMBL, respectively.
Protein Name
The common
name given to a protein, that identifies its
function or specifies its features.
Length
Number of amino acid
residues in the peptide or protein.
Organism Name
The genus and species of the source organism from which the sequence originated. Links to NCBI taxonomy information are provided.
PIRSF ID
If a protein belongs to a PIRSF family, then this column will display the corresponding family identifier. Click on the ID to retrieve the PIRSF report.
Match
Range
This column displays in red the peptide query within the sequence.
6- GO Slim
GO slims are smaller versions of the Gene Ontologies containing a subset of the terms in the whole GO. They give a broad overview of the ontology content without the detail of the specific fine grained terms. GO slims are particularly useful for giving a summary of the results of GO annotation of a genome or proteome when broad classification of gene product function is required.
You can view the GO slim terms for Biological Function, Component, and Process by selecting the “Show GO Slim” button in the analysis tool bar. You can then view statistics for the individual ontologies (Function, Component, and Function) by checking entries of interest and selecting the ontology to show (for example, function in this example). Follow the GO ID links to learn more about the GO term.
7- Show match list
Shows a table mapping your query IDs with the UniProtKB/UniParc IDs.
Batch Retrieval Output in PIRSF Database
Retrieval of PIRSFs using as queries UniProtKB ACs P51375, P09831 and Q05755
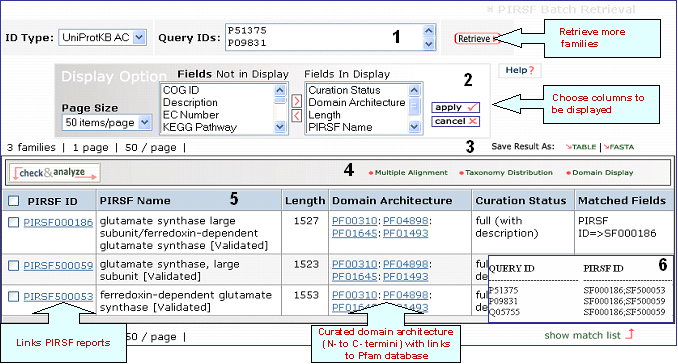
1- Retrieval Box
This box shows your query ID and also allows you to perform a new retrieval
2- Display Options
Depending on your specific need you can
choose the columns to be displayed. To do this, click on the "Display Option" button, select the
relevant field(s) in the "Fields Not in Display"
list and transfer them to the "Fields in
Display" list via the ">" button. Conversely,
columns can be removed from display. Finally, click on "apply"
for the changes to take effect.
3- Save Results
As
The output can be saved to the
user's local computer. The results will be saved
for selected entries or, if no proteins are
selected, for all entries. Clicking "Table" will
save the displayed columns as a tab-delimited text
file, which may be imported into a spreadsheet for
easier viewing or analysis. Clicking "FASTA" will
save the IDs and sequences in FASTA
format.
4- Analyze: Multiple Alignment, Taxonomic Distribution and
Domain Display
Retrieved entries can be
further analyzed using the sequence analysis
programs available in the Results page. First,
select the PIRSF(s) using the checkboxes on the
left side of the table, then click the
corresponding analysis tool. Click on the "Multiple
Alignment" button to generate a ClustalW alignment
and neighbor-joining tree for the members of the selected PIRSFs. If one PIRSF is selected, then alignment of all its seed members will be performed (see sample output for PIRSF000186). But if many PIRSFs are selected, then alignment of the representative sequences will be displayed. Click on Taxonomic Distribution to look at the number of members of a PIRSF present in the different taxa. You can perform this function on one or multiple PIRSFs. If only one PIRSF is selected, the taxonomic distribution for the parent family and the children (if any) will be displayed. See sample output for PIRSF000186.
Domain display option, shows PFam domains (if
present) in a graphical format. See sample output or annotated output for PIRSF000186.
5- Results Display
Results of
the search are displayed in a table.
PIRSF ID
This column will display the corresponding family identifier. Click on the ID to retrieve the PIRSF report. The icon below the PIRSF ID states the family level, namely homeomorphic family (HFam), subfamily (SubFam), and superfamily (SuperFam). Clicking on this icon will bring the DAG view of PIRSF Hierarchy with the PFam domain at the higher level.
PIRSF Name
The
name given to the family that identifies its
function or specifies its features. Depending on the curation status, this name is assigned automatically (no manual curation) or manually (full curation). If the family is curated, it may display a tag next to the name indicating its status, namely validated (there is experimental data supporting PIRSF name), tentative (there is no conclusive evidence) or predicted (predicted by computational methods).
Length
It is the protein average length of the PIRSF members.
Domain Architecture
It represents the curated information regarding present Pfam domains in the protein family. Pfam domains are listed in order from N- to C- terminus separated by semi-colons. In a few cases, domains are separated by a dash indicating the presence of inserted domains.
Numbers in parenthesis indicate the repetition of a domain. There is a particular syntax for this feature. For example, PF11111(1-3) allows for 1 to 3 copies of PF11111, whereas PF11111(2-) allows for any number of domains above 1 (2 or more). However, PF11111(0,2) allows for none or two copies of this domain.
Curation Status
Uncurated: Computer-generated
protein clusters, not
curated.
Preliminary: Membership and
domain architecture of protein families determined
by manual curation.
Full:Protein family
name with accompanying references (when
available), and sometimes brief descriptions,
provided after thorough manual curation.
Matched
Fields
The field(s) matched by the
query.
6- Show match list
Shows a table mapping your query IDs to the corresponding PIRSF.
BLAST Search Help
What is BLAST?
The Basic Local
Alignment Search Tool (BLAST) allows rapid
sequence comparison that optimizes the
high-scoring segment pair (HSP), a measure of
local similarity. For more information visit NCBI-BLAST.
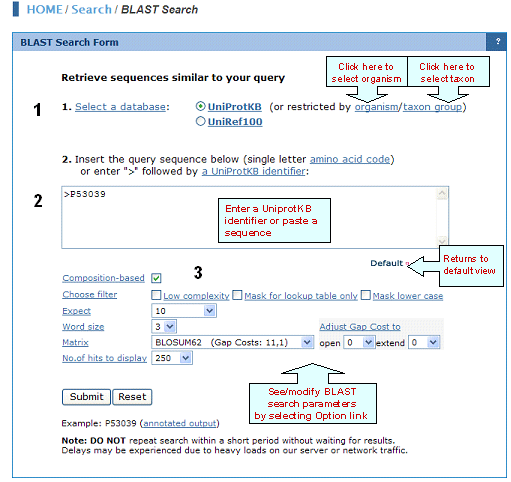
1. Select a Database
BLAST search can be performed against: UniProtKB is the central hub for the collection of functional information on proteins, with accurate, consistent, and rich annotation. It consists of two sections: a section containing manually-annotated records with information extracted from literature and curator-evaluated computational analysis (UniProtKB/Swiss-Prot), and a section with computationally analyzed records that await full manual annotation (UniProtKB/TrEMBL).
A subset of UniProtKB entries belonging to a certain organism or taxon group.
UniRef100 provides clustered sets of sequences at 100 % from UniProt Knowledgebase (including splice variants and isoforms) and selected UniParc records (mainly from PDB, Refseq and Ensembl databases), in order to obtain complete coverage of sequence space while hiding redundant sequences (but not their descriptions) from view. It combines identical sequences and sub-fragments with 11 or more residues (from any organism) into a single UniRef entry, displaying the sequence of a representative protein, the accession numbers of all the merged UniProtKB entries, and links to the corresponding UniProtKB and UniParc records.
.
2. BLAST Search Format
The
BLAST 'Search' input area accepts:
">" followed by a UniProtKB sequence identifier. Spaces between letters in
the input are not allowed, although spaces before
or after the identifier are allowed.
Raw amino
acid sequence format:MSEPQRLFFAIDLPAEIREQIIHWRATHFPPEAGRPVAADNLHLTLAFLGEVS
AEKEKALSLLAGRIRQPGFTLTLDDAGQWLRSRVVWLGMRQPPRGLIQLAN
MLRSQAARSGCFQSNRPFHPHITLLRDASEAVTIPPPGFNWSYAVTEFTLYA
SSFARGRTRYTPLKRWALTQ
The query
sequence intersperse with numbers and/or spaces, such as:
1 msepqrlffa idlpaeireq iihwrathfp peagrpvaad nlhltlaflg evsaekekal
61 sllagrirqp gftltlddag qwlrsrvvwl gmrqpprgli qlanmlrsqa arsgcfqsnr
121 pfhphitllr daseavtipp pgfnwsyavt eftlyassfa rgrtrytplk rwaltq
Sequences are
expected to be represented in the standard
IUB/IUPAC amino acid codes, with these exceptions:
lower-case letters are accepted and are mapped
into upper-case; U and * are acceptable letters
(see table).
Numerical digits in the query sequence are
automatically removed.
BLAST
Options
BLAST is performed using the BLOSUM62 matrix with default values for gap opening and extension cost. However, the
following parameters can be set:
-Composition-based Statistics
BLAST permits calculated E-values to take into account the amino acid composition of the individual database sequences involved in reported alignments. This improves E-value accuracy, thereby reducing the number of false positive results. The improved statistics are achieved with a scaling procedure that in effect employs a slightly different scoring system for each database sequence. As a result, raw BLAST alignment scores in general will not correspond precisely to those implied by any standard substitution matrix. Furthermore, identical alignments can receive different scores, based upon the compositions of the sequences they involve.
-Filter
Low-complexity
Masks off segments of the query sequence that have low compositional complexity, as determined by the SEG program of Wootton & Federhen (Computers and Chemistry, 1993). Filtering can eliminate statistically significant but biologically uninteresting reports from the BLAST output (e.g., hits against common acidic-, basic- or proline-rich regions), leaving the more biologically interesting regions of the query sequence available for specific matching against database sequences. Filtering is only applied to the query sequence, not to database sequences.
Mask for Lookup Table Only
This option masks only for purposes of constructing the lookup table used by BLAST. BLAST searches consist of two phases, finding hits based upon a lookup table and then extending them. The option to "Mask for lookup table only" masks only for the lookup table so that no hits are found based upon low-complexity sequence. The BLAST extensions are performed without masking and so they can be extended through low-complexity sequence. This option is still experimental and may change in the near future.
Mask Lower Case
With this option selected you can cut and paste a FASTA sequence in upper case characters and denote areas you would like filtered with lower case. This allows you to customize what is filtered from the sequence during the comparison to the BLAST databases.
-Expect
The Expect threshold establishes a statistical significance threshold for reporting database sequence matches. The default value is 10, meaning that 10 matches are expected to be found merely by chance. Lower Expect thresholds are more stringent, leading to fewer chance matches being reported. Increasing the expected threshold shows less stringent matches and is recommended when performing searches with short sequences as a short query is more likely to occur by chance in the database than a longer one, so even a perfect match (no gaps) can have low statistical significance and may not be reported. Increasing the Expect threshold allows you to look farther down in the hit list and see matches that would normally be discarded because of low statistical significance.
-Word Size
The word size indicates the length of the initial sequence that must be matched between the database and the query sequence.
-Matrix
A key element in evaluating the quality of a pairwise sequence alignment is the "substitution matrix", which assigns a score for aligning any possible pair of residues. The matrix used in a BLAST search can be changed depending on the type of sequences you are searching with. The user may choose from a list of matrices that cover various evolutionary constraints (more information can be found in a description of BLAST scoring matrices). For each matrix, a default matrix-dependent gap cost is displayed. Gap costs are described below.
-Matrix-dependent Gap Cost
The pull down menu shows the Gap Costs (penalty to open gap and penalty to extend gap). There are a limited number of options for these parameters. Increasing the Gap Costs will result in alignments that decrease the number of Gaps introduced. The gap open penalty is the score taken away for the initiation of a gap in a sequence. To make the match more significant the user can try making the gap penalty larger. The gap extension penalty is added to the gap open penalty for each residue in the gap, effectively penalizing longer gaps. If the user does not like long gaps, they can increase the extension gap penalty. Usually one would expect a few long gaps rather than many short gaps, so the gap extension penalty should be lower than the gap penalty. An exception is where one or both sequences are single reads with possible sequencing errors, in which case you would expect many single base gaps. The user can get this result by setting the gap open penalty to zero (or very low) and using the gap extension penalty to control gap scoring.
-Adjust Gap Costs
Alignments between sequences are often optimized by allowing gaps within one or both sequences. Like mismatches between aligned residues, gaps have a "cost" associated with them. There are separate penalties to open and to extend gaps. Increasing the Gap Costs will result in alignments that decrease the number and size of Gaps introduced. The Gap Open cost (or Gap Existence cost) is the score taken away for the initiation of a gap in a sequence. To make the match more significant the user can try making this gap penalty larger. The Gap Extend cost is added to the Gap Open cost for each residue in the gap, effectively penalizing longer gaps. The user can therefore select against long gaps by increasing this penalty. Usually one would expect a few long gaps rather than many short gaps, so the Gap Extend cost should be lower than the Gap Open cost. The Gap Costs can be adjusted relative to the default value using the pull down menu.
-Number of Hits to Display
Restricts the number of BLAST hits of matching sequences that will be reported.
-Alignment
Aligns your query sequence and database matches in pairs. Matches are connected with a "|" symbol. Mismatches are opposed with a space. Gaps are introduced with a "-" symbol.
References
Wootton JC, and Federhen S (1993) Statistics of local complexity in amino acid sequences and sequence databases. Computers and Chemistry 17:149-163.
BLAST Results Help
A sample output for a BLAST search against the UniProtKB database is shown below
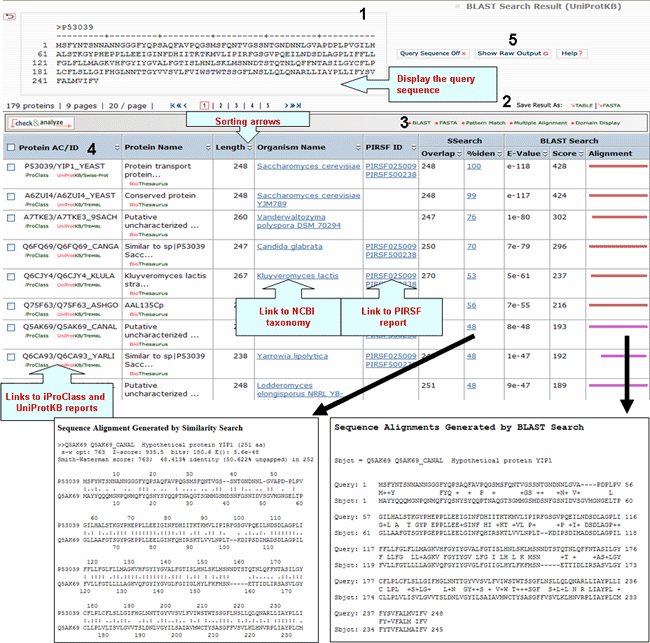
1- Query Sequence on
Click on this button to display the query sequence. Conversely, click it again to hide it.
2- Save Results As
Search results can be saved to the user's local computer. The results will be saved for selected entries or, if no proteins are selected, for all entries. Clicking "Table" will save the displayed columns as a tab-delimited text file, which may be imported into a spreadsheet for easier viewing or analysis. Clicking "FASTA" will save the IDs and sequences in FASTA format.
3- BLAST,
FASTA, Pattern Match, Multiple Alignment and
Domain Display
Retrieved entries can be
further analyzed using the sequence analysis
programs available in the Results page. First,
select the protein(s) using the checkboxes on the
left side of the table, then click the
corresponding analysis tool. Click "BLAST" or
"FASTA" button, and a new query page will be
displayed, along with the parameters that were
selected in the initial search. Click "Pattern
Match" to search against the PROSITE database. For
multiple alignment, check at least 2 proteins (but
no more than 70), then click the "Multiple
Alignment" button. This will open the Multiple alignment form from which you can select one of the alignment programs: ClustalW, T-Coffee or Muscle. The result page will display the alignment and alignment viewer. For ClustalW and T-Coffee, the neighbor-joining tree and alignment can be viewed, edited and saved using either PIR-TAV viewer or JalView. For Muscle only Jalview is available.
Domain display option, shows PFam domains (if
present) in graphical format.
4- Results
Display
Sort Columns
Columns can be
sorted by the corresponding values by clicking on
the arrow next to the column title. By default
the table is sorted by Score.
The results from the search are displayed
in a table with the following default columns:
Protein AC/ID
The
Protein AC/ID refers to the UniProtKB accession number and ID, respectively. Below these identifiers, you may choose either the iProClass or the UniProtKB view of the protein report. The source of the UniProtKB sequence is shown as UniProtKB/Swiss-Prot or UniProtKB/TrEMBL if the protein sequence is from the Swiss-Prot or TrEMBL section of UniProtKB, respectively.
Protein
Name
The common name given to a protein,
that identifies its function or specifies its
features.
Length
Number of amino acid
residues in the peptide or protein.
Organism Name
The genus and species of the source organism from which the sequence originated. Links to NCBI taxonomy information are provided.
PIRSF ID
If a protein belongs to a PIRSF family, then this column will display the corresponding family identifier. Click on the ID to retrieve the PIRSF report (see annotated output).
BLAST
Sequence Similarity Columns
There are three
columns related to BLAST results. The
Alignment column, in the extreme right, shows the
BLAST results in graphical format. The top
bar represents the query sequence. The bars below
show the region(s) on other sequences matched to
the query sequence. The bar color indicates the
magnitude of the BLAST score. Click on one of
these bars to see its BLASTalignment paired
with the query sequence.
SSearch Columns
SSearch is
a pairwise implementation of the Smith-Waterman
alignment algorithm. When two sequences are
aligned, only the shared region is shown. Within
the shared region, amino acid residues from one or
both sequences can be aligned with either amino
acids or gaps from the other sequence. The total
length of the shared region, including gaps, is
represented under the Overlap column. The
percentage of identical residues in the alignment
is given under %iden. Clicking on that number
displays the SSearch full-length
alignment.
5- Show Raw Output
The report consists of three major sections: (1) the header, which contains information about the query sequence, the database searched. (2) the one-line descriptions of each database sequence found to match the query sequence; these provide a quick overview for browsing; (3) the alignments for each database sequence matched (there may be more than one alignment for a database sequence it matches).
In the one-line descriptions in the BLAST report, each line is composed of five fields: (a) UniProtKB accession number (b) UniProtKB ID, (c) the protein name. This line is often truncated in the one-line descriptions to keep the display compact; (d) the alignment score in bits. Higher scoring hits are found at the top of the list; and (e) the E-value, which provides an estimate of statistical significance. Adapted from The NCBI Handbook.
A sample output for a BLAST search against the UniRef100 database is shown below
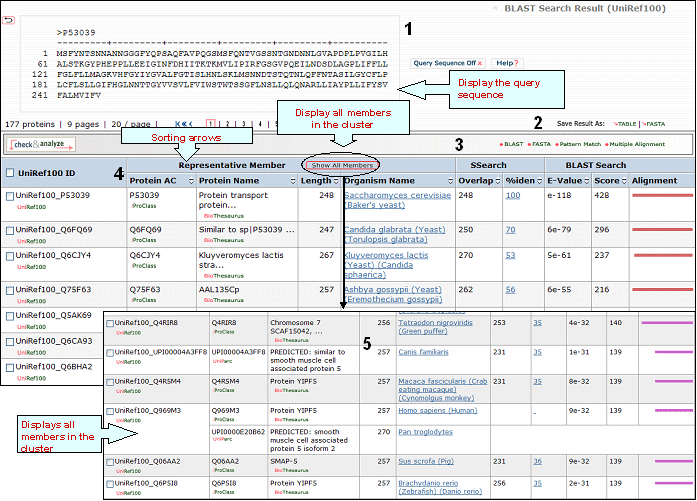
1- Query Sequence on
Click on this button to display the query sequence. Conversely, click it again to hide it.
2- Save Results As
Search results can be saved to the user's local computer. The results will be saved for selected entries or, if no proteins are selected, for all entries. Clicking "Table" will save the displayed columns as a tab-delimited text file, which may be imported into a spreadsheet for easier viewing or analysis. Clicking "FASTA" will save the IDs and sequences in FASTA format.
3- BLAST,
FASTA, Pattern Match, Multiple Alignment and
Domain Display
Retrieved entries can be
further analyzed using the sequence analysis
programs available in the Results page. First,
select the protein(s) using the checkboxes on the
left side of the table, then click the
corresponding analysis tool. Click "BLAST" or
"FASTA" button, and a new query page will be
displayed, along with the parameters that were
selected in the initial search. Click "Pattern
Match" to search against the PROSITE database. For
multiple alignment, check at least 2 proteins (but
no more than 70), then click the "Multiple
Alignment" button. This will open the Multiple alignment form from which you can select one of the alignment programs: ClustalW, T-Coffee or Muscle. The result page will display the alignment and alignment viewer. For ClustalW and T-Coffee, the neighbor-joining tree and alignment can be viewed, edited and saved using either PIR-TAV viewer or JalView. For Muscle only Jalview is available.
4- Results
Display
Sort Columns
Columns can be
sorted by the corresponding values by clicking on
the arrow next to the column title. By default
the table is sorted by Score.
BLAST results are calculated for the representative member of each cluster, you may see the other members (but no BLAST results) by clicking on the "Show all members" logo. Results are shown in a table with the following default columns:
UniRef100 ID
It refers to the cluster identifier. Below this there is a link to the UniRef100 report with information on the UniRef100 cluster members, as well as links to the UniRef50 and UniRef90 clusters.
Protein AC
The
Protein AC refers to the UniProtKB accession or UniParc ID. Below these identifiers, you may choose either the iProClass or the UniParc view of the protein report, respectively.
Protein
Name
The common name given to a protein,
that identifies its function or specifies its
features.
Length
Number of amino acid
residues in the peptide or protein.
Organism Name
The genus and species of the source organism from which the sequence originated. Links to NCBI taxonomy information are provided.
BLAST
Sequence Similarity Columns
There are three
columns related to BLAST results. The
Alignment column, in the extreme right, shows the
BLAST results in graphical format. The top
bar represents the query sequence. The bars below
show the region(s) on other sequences matched to
the query sequence. The bar color indicates the
magnitude of the BLAST score. Click on one of
these bars to see its BLASTalignment paired
with the query sequence.
SSearch Columns
SSearch is
a pairwise implementation of the Smith-Waterman
alignment algorithm. When two sequences are
aligned, only the shared region is shown. Within
the shared region, amino acid residues from one or
both sequences can be aligned with either amino
acids or gaps from the other sequence. The total
length of the shared region, including gaps, is
represented under the Overlap column. The
percentage of identical residues in the alignment
is given under %iden. Clicking on that number
displays the SSearch full-length
alignment.
5- Show All Members
By clicking this logo you will be able to see all sequences belonging to individual clusters.
FASTA Search Help
What is FASTA?
FASTA can be
used to search sequence databases, evaluate
similarity scores, and identify periodic
structures based on local sequence similarity. The
FASTA program can compare a protein sequence to a
DNA sequence database by translating the DNA
database as it is searched. This search engine
displays FASTA results (up to 200 matches), using
the FASTA program (Pearson and
Lipman, 1988) with default settings.
Select a Database
Perform FASTA search against: UniProtKB is the central hub for the collection of functional information on proteins, with accurate, consistent, and rich annotation. It consists of two sections: a section containing manually-annotated records with information extracted from literature and curator-evaluated computational analysis (UniProtKB/Swiss-Prot), and a section with computationally analyzed records that await full manual annotation (UniProtKB/TrEMBL).
A subset of UniProtKB entries belonging to a certain organism or taxon group.
UniRef100 provides clustered sets of sequences at 100 % from UniProt Knowledgebase (including splice variants and isoforms) and selected UniParc records (mainly from PDB, Refseq and Ensembl databases), in order to obtain complete coverage of sequence space while hiding redundant sequences (but not their descriptions) from view. It combines identical sequences and sub-fragments with 11 or more residues (from any organism) into a single UniRef entry, displaying the sequence of a representative protein, the accession numbers of all the merged UniProtKB entries, and links to the corresponding UniProtKB and UniParc records.
FASTA Search Format
The FASTA 'Search' input area accepts:
">" followed by a UniProtKB
sequence identifier. Spaces between letters
in the input are not allowed, although spaces
before or after the identifier are allowed.
Raw amino acid sequence format:MSEPQRLFFAIDLPAEIREQIIHWRATHFPPEAGRPVAADNLHLTLAFLGEVS
AEKEKALSLLAGRIRQPGFTLTLDDAGQWLRSRVVWLGMRQPPRGLIQLAN
MLRSQAARSGCFQSNRPFHPHITLLRDASEAVTIPPPGFNWSYAVTEFTLYA
SSFARGRTRYTPLKRWALTQ
The query sequence intersperse with numbers and/or spaces, such as:
1 msepqrlffa idlpaeireq iihwrathfp peagrpvaad nlhltlaflg evsaekekal
61 sllagrirqp gftltlddag qwlrsrvvwl gmrqpprgli qlanmlrsqa arsgcfqsnr
121 pfhphitllr daseavtipp pgfnwsyavt eftlyassfa rgrtrytplk rwaltq
Sequences are expected to be represented in
the standard IUB/IUPAC amino acid codes, with
these exceptions: lower-case letters are accepted
and are mapped into upper-case; U and * are
acceptable letters (see table)
Numerical digits in the query sequence are
automatically removed.
FASTA Options
-Expect
The
Expect (E-value) threshold establishes a
statistical significance threshold for reporting
database sequence matches. The default value is
0.0001, meaning that 0.0001 matches are expected
to be found merely by chance. Lower Expect
thresholds are more stringent, leading to fewer
chance matches being reported. Increasing the
expected threshold shows less stringent matches
and is recommended when performing searches with
short sequences as a short query is more likely to
occur by chance in the database than a longer one,
so even a perfect match (no gaps) can have low
statistical significance and may not be reported.
Increasing the Expect threshold allows you to look
farther down in the hit list and see matches that
would normally be discarded because of low
statistical significance.
References
Pearson WR, and
DJ Lipman (1988) Improved tools for biological
sequence comparison. Proc. Natl. Acad. Sci. USA
85(8): 2444-2448.
FASTA Results Help
A sample output for a BLAST search against the UniProtKB database is shown below
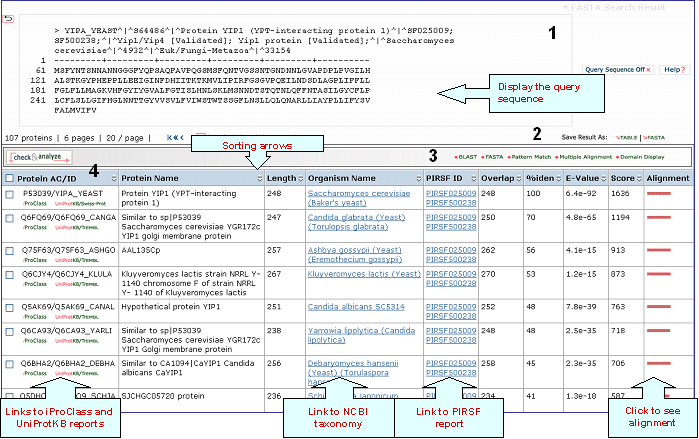
1- Query Sequence on
Click on this button to display the query sequence. Conversely, click it again to hide it.
2- Save Results As
Search results can be saved to the user's local computer. The results will be saved for selected entries or, if no proteins are selected, for all entries. Clicking "Table" will save the displayed columns as a tab-delimited text file, which may be imported into a spreadsheet for easier viewing or analysis. Clicking "FASTA" will save the IDs and sequences in FASTA format.
3- BLAST,
FASTA, Pattern Match, Multiple Alignment and
Domain Display
Retrieved entries can be
further analyzed using the sequence analysis
programs available in the Results page. First,
select the protein(s) using the checkboxes on the
left side of the table, then click the
corresponding analysis tool. Click "BLAST" or
"FASTA" button, and a new query page will be
displayed, along with the parameters that were
selected in the initial search. Click "Pattern
Match" to search against the PROSITE database. For
multiple alignment, check at least 2 proteins (but
no more than 70), then click the "Multiple
Alignment" button. This will open the Multiple alignment form from which you can select one of the alignment programs: ClustalW, T-Coffee or Muscle. The result page will display the alignment and alignment viewer. For ClustalW and T-Coffee, the neighbor-joining tree and alignment can be viewed, edited and saved using either PIR-TAV viewer or JalView. For Muscle only Jalview is available.
Domain display option, shows PFam domains (if
present) in graphical format.
4- Results
Display
Sort Columns
Columns can be
sorted by the corresponding values by clicking on
the arrow next to the column title. By default
the table is sorted by Score.
The results from the search are displayed
in a table with the following default columns:
Protein AC/ID
The
Protein AC/ID refers to the UniProtKB accession number and ID, respectively. Below these identifiers, you may choose either the iProClass or the UniProtKB view of the protein report. The source of the UniProtKB sequence is shown as UniProtKB/Swiss-Prot or UniProtKB/TrEMBL if the protein sequence is from the Swiss-Prot or TrEMBL section of UniProtKB, respectively.
Protein
Name
The common name given to a protein,
that identifies its function or specifies its
features.
Length
Number of amino acid
residues in the peptide or protein.
Organism Name
The genus and species of the source organism from which the sequence originated. Links to NCBI taxonomy information are provided.
PIRSF ID
If a protein belongs to a PIRSF family, then this column will display the corresponding family identifier. Click on the ID to retrieve the PIRSF report (see annotated output).
Sequence Similarity Columns
There are three
columns related to FASTA results. The
Alignment column, in the extreme right, shows the
FASTA results in graphical format. The top
bar represents the query sequence. The bars below
show the region(s) on other sequences matched to
the query sequence. The bar color indicates the
magnitude of the FASTA score. Click on one of
these bars to see its FASTA alignment paired
with the query sequence.
Related Sequence HelpRetrieve
related sequences based on pre-computed BLAST
results, therefore you will be able to have a glance at proteins similar to your query, significantly faster than running BLAST. This procedure is performed approximately every three months. Enter the UniProtKB
sequence identifier and click "Search" button.
Sample output for UniProtKB P53039

1- E-value display
Select the E-value cut-off to display your results.
2- Query Sequence on
Click on this button to display the query sequence. Conversely, click it again to hide it.
3- Save Results As
Search results can be saved to the user's local computer. The results will be saved for selected entries or, if no proteins are selected, for all entries. Clicking "Table" will save the displayed columns as a tab-delimited text file, which may be imported into a spreadsheet for easier viewing or analysis. Clicking "FASTA" will save the IDs and sequences in FASTA format.
4- BLAST,
FASTA, Pattern Match, Multiple Alignment and
Domain Display
Retrieved entries can be
further analyzed using the sequence analysis
programs available in the Results page. First,
select the protein(s) using the checkboxes on the
left side of the table, then click the
corresponding analysis tool. Click "BLAST" or
"FASTA" button, and a new query page will be
displayed, along with the parameters that were
selected in the initial search. Click "Pattern
Match" to search against the PROSITE database. For
multiple alignment, check at least 2 proteins (but
no more than 70), then click the "Multiple
Alignment" button. This will open the Multiple alignment form from which you can select one of the alignment programs: ClustalW, T-Coffee or Muscle. The result page will display the alignment and alignment viewer. For ClustalW and T-Coffee, the neighbor-joining tree and alignment can be viewed, edited and saved using either PIR-TAV viewer or JalView. For Muscle only Jalview is available.
Domain display option, shows PFam domains (if
present) in graphical format.
5- Results
Display
Sort Columns
Columns can be
sorted by the corresponding values by clicking on
the arrow next to the column title. By default
the table is sorted by Score.
The results from the search are displayed
in a table with the following default columns:
Protein AC/ID
The
Protein AC/ID refers to the UniProtKB accession number and ID, respectively. Below these identifiers, you may choose either the iProClass or the UniProtKB view of the protein report. The source of the UniProtKB sequence is shown as UniProtKB/Swiss-Prot or UniProtKB/TrEMBL if the protein sequence is from the Swiss-Prot or TrEMBL section of UniProtKB, respectively.
Protein
Name
The common name given to a protein,
that identifies its function or specifies its
features.
Length
Number of amino acid
residues in the peptide or protein.
Organism Name
The genus and species of the source organism from which the sequence originated. Links to NCBI taxonomy information are provided.
PIRSF ID
If a protein belongs to a PIRSF family, then this column will display the corresponding family identifier. Click on the ID to retrieve the PIRSF report (see annotated output).
Sequence Similarity Columns
There are three
columns related to BLAST results. The
Alignment column, in the extreme right, shows the
BLAST results in graphical format. The top
bar represents the query sequence. The bars below
show the region(s) on other sequences matched to
the query sequence. The bar color indicates the
magnitude of the BLAST score. Click on one of
these bars to see its alignment paired
with the query sequence.
Peptide Match
Find an exact match for a peptide sequence query in the selected database.
You can perform the search against: UniProtKB is the central hub for the collection of functional information on proteins, with accurate, consistent, and rich annotation. It consists of two sections: a section containing manually-annotated records with information extracted from literature and curator-evaluated computational analysis (UniProtKB/Swiss-Prot), and a section with computationally analyzed records that await full manual annotation (UniProtKB/TrEMBL).
A subset of UniProtKB entries belonging to a certain organism or a set of organisms.
Peptide Match Result page

1- Query Peptide
Show the query peptide and allow user to do a new search by submitting a new query peptide.
2- Save Results
As
The output can be saved to the
user's local computer. The results will be saved
for selected entries or, if no proteins are
selected, for all entries. Clicking "Table" will
save the displayed columns as a tab-delimited text
file, which may be imported into a spreadsheet for
easier viewing or analysis. Clicking "FASTA" will
save the IDs and sequences in FASTA
format.
3- Analyze: BLAST,
FASTA, Pattern Match, Multiple Alignment and
Domain Display
Retrieved entries can be
further analyzed using the sequence analysis
programs available in the Results page. First,
select the protein(s) using the checkboxes on the
left side of the table, then click the
corresponding analysis tool. Click "BLAST" or
"FASTA" button, and a new query page will be
displayed, along with the parameters that were
selected in the initial search. Click "Pattern
Match" to search against the PROSITE database. For
multiple alignment, check at least 2 proteins (but
no more than 70), then click the "Multiple
Alignment" button. This will open the Multiple alignment form from which you can select one of the alignment programs: ClustalW, T-Coffee or Muscle. The result page will display the alignment and alignment viewer. For ClustalW and T-Coffee, the neighbor-joining tree and alignment can be viewed, edited and saved using either PIR-TAV viewer or JalView. For Muscle only Jalview is available.
Domain display option, shows PFam domains (if
present) in graphical format.
4- Results Display
Results of
the search are displayed in a table.
Protein AC/ID
The
Protein AC/ID refers to the UniProtKB or UniRef100 identifiers. Below these numbers, you may choose either the iProClass or the UniProtKB/UniRef100 view of each protein report. The source of the UniProtKB sequence is shown as UniProtKB/Swiss-Prot or UniProtKB/TrEMBL if the protein sequence is from Swiss-Prot or TrEMBL, respectively.
Protein Name
The common
name given to a protein, that identifies its
function or specifies its features.
Length
Number of amino acid
residues in the peptide or protein.
Organism Name
The genus and species of the source organism from which the sequence originated. Links to NCBI taxonomy information are provided.
PIRSF ID
If a protein belongs to a PIRSF family, then this column will display the corresponding family identifier. Click on the ID to retrieve the PIRSF report (see annotated output).
Match
Range
This column displays in red the peptide query within the sequence.
5- Multiple organisms
Click to show the taxonomy IDs of matched organisms.
6- Browse by taxonomy group
Click to show the taxonomy groups of matched sequences as shown below.

Batch Peptide Match
Find the exact match for a set of query peptide sequences in the selected database:
You can perform the search against: UniProtKB is the central hub for the collection of functional information on proteins, with accurate, consistent, and rich annotation. It consists of two sections: a section containing manually-annotated records with information extracted from literature and curator-evaluated computational analysis (UniProtKB/Swiss-Prot), and a section with computationally analyzed records that await full manual annotation (UniProtKB/TrEMBL).
A subset of UniProtKB entries belonging to a certain organism or a set of organisms.
The batch peptide match results can be retrieved as a text file for further analysis. The match results are organised based on the query peptide sequences.
Pattern Search
A pattern is a formula (regular expression) that
represents the conserved region of a group of
related proteins. Once constructed, the pattern is
used by a pattern-matching program to find
possible occurrences of the conserved region in
the sequence database.
PROSITE is a database that contains patterns and profiles specific for more than a thousand protein families or domains. Each of these signatures comes with documentation providing background information on the structure and function of these proteins.
Identification of patterns in a protein or group of proteins can help in assessing protein function or predict a certain post-translational modification. However, pattern search results should be further investigated, since due to the nature of the approach the patterns are often too specific (many false negatives) or insufficiently selective (high probability of occurrence). As is the case, for example, for the PROSITE pattern:
PS00001
ID ASN_GLYCOSYLATION; PATTERN.
DE N-glycosylation site.
PA N-{P}-[ST]-{P}
This pattern can be found in most proteins, however, N-glycosylation is a modification that takes place in the Endoplasmic Reticulum during synthesis of membrane and secreted proteins.
Pattern search at PIR allows:
The search of PROSITE patterns (note that we only include patterns, profiles are excluded) in a query sequence, entering the single amino acid code sequence or its
unique ID
The search of a specific PROSITE or user-defined pattern
against one of the following sequence database:
(i)UniProtKB is the central hub for the collection of functional information on proteins, with accurate, consistent, and rich annotation. It consists of two sections: a section containing manually-annotated records with information extracted from literature and curator-evaluated computational analysis (UniProtKB/Swiss-Prot), and a section with computationally analyzed records that await full manual annotation (UniProtKB/TrEMBL).
(ii) A subset of UniProtKB entries belonging to a certain organism or taxon group.
(iii) UniRef100 provides clustered sets of sequences at 100 % from UniProt Knowledgebase (including splice variants and isoforms) and selected UniParc records (mainly from PDB, Refseq and Ensembl databases), in order to obtain complete coverage of sequence space while hiding redundant sequences (but not their descriptions) from view. It combines identical sequences and sub-fragments with 11 or more residues (from any organism) into a single UniRef entry, displaying the sequence of a representative protein, the accession numbers of all the merged UniProtKB entries, and links to the corresponding UniProtKB and UniParc records.
How to write a protein
pattern
Follow the example below to build
the protein pattern.
[LIVM]-[VIC]-x(2) -G-[DENQTA]-x-[GAC]-x(2) -[LIVMFY](4)-x(2)-G
- Use capital letters for amino acid residues
and put a "-" between two amino acids (not
required).
- Use "[…] for a choice of
multiple amino acids in a particular position.
- [LIVM] means that L, I,
V, or M can be in the first
position.
- Use "{…}" to exclude amino acids.
- {CF} means C and F should not be in that
particular position
- Use "x" for a
position that can be any amino acid.
- Use "(n)", where
n is a number, for multiple positions.
- x (3) is the same as "xxx"
- Use "(n1,n2)" for multiple or variable
positions.
- " x (1,4) represents "x" or "xx" or "xxx" or
"xxxx"
- Use the symbol ">" at the beginning or
end of the pattern to require the pattern to
match the N or C terminus.
- ">MDEL" finds only sequences that start
with MDEL
- "DEL>" finds only sequences that end with
DEL
[LIVM]-[VIC]-x(2) -G-[DENQTA]-x-[GAC]-x(2) -[LIVMFY](4)-x(2)-G Illustrates a 17 amino acid peptide that
has: an L, I, V, or M at position 1; a V, I, or C
at position 2; any residue at positions 3 and 4; a
G at position 5 and so on ….
Note: You can
also write the above pattern as:
[LIVM] [VIC] x
(2) G [DENQTA] x [GAC] x (2) [LIVMFY] (4) x (2)
G
Result for proteins with PROSITE pattern PS00888 (Cyclic nucleotide-binding domain signature 1)

1- Save Results
As
The output can be saved to the
user's local computer. The results will be saved
for selected entries or, if no proteins are
selected, for all entries. Clicking "Table" will
save the displayed columns as a tab-delimited text
file, which may be imported into a spreadsheet for
easier viewing or analysis. Clicking "FASTA" will
save the IDs and sequences in FASTA
format.
2- BLAST,
FASTA, Pattern Match, Multiple Alignment and
Domain Display
Retrieved entries can be
further analyzed using the sequence analysis
programs available in the Results page. First,
select the protein(s) using the checkboxes on the
left side of the table, then click the
corresponding analysis tool. Click "BLAST" or
"FASTA" button, and a new query page will be
displayed, along with the parameters that were
selected in the initial search. Click "Pattern
Match" to search against the PROSITE database. For
multiple alignment, check at least 2 proteins (but
no more than 70), then click the "Multiple
Alignment" button. This will open the Multiple alignment form from which you can select one of the alignment programs: ClustalW, T-Coffee or Muscle. The result page will display the alignment and alignment viewer. For ClustalW and T-Coffee, the neighbor-joining tree and alignment can be viewed, edited and saved using either PIR-TAV viewer or JalView. For Muscle only Jalview is available.
Domain display option, shows PFam domains (if
present) in graphical format.
3- Results Display
Results of
the search are displayed in a table. Columns can be
sorted by the corresponding values by clicking on
the arrow next to the column title.
Protein AC/ID
The
Protein AC/ID refers to the UniProtKB accesion and identifier, respectively. Below these, you may choose either the iProClass or the UniProtKB view of each protein report. The source of the UniProtKB sequence is shown as UniProtKB/Swiss-Prot or UniProtKB/TrEMBL if the protein sequence is from Swiss-Prot or TrEMBL, respectively.
Protein Name
The common
name given to a protein, that identifies its
function or specifies its features.
Length
Number of amino acid
residues in the peptide or protein.
Organism Name
The genus and species of the source organism from which the sequence originated. Links to NCBI taxonomy information are provided.
PIRSF ID
If a protein belongs to a PIRSF family, then this column will display the corresponding family identifier. Click on the ID to retrieve the PIRSF report (see annotated output).
Match
Range
This column displays the sequence range for the query pattern.
Partial result for PROSITE patterns in UniProtKB O05689

Multiple Alignment Help
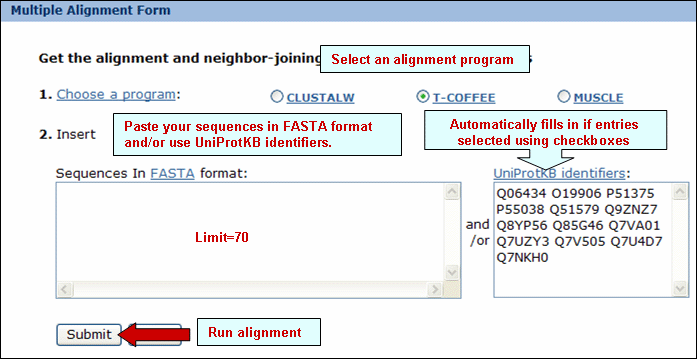
Select the alignment program: ClustalW, T-Cofee or Muscle. Then, enter multiple sequences (no more than 70) with
their corresponding ID lines in FASTA
format, or enter multiple UniProtKB
IDs separated by lines, commas or spaces. Then
click the "Submit" button.
The result page will display the alignment and alignment viewer. For ClustalW and T-Coffee, the neighbor-joining tree and alignment can be viewed, edited and saved using either PIR-TAV viewer or JalView (see below). For Muscle only Jalview is available.
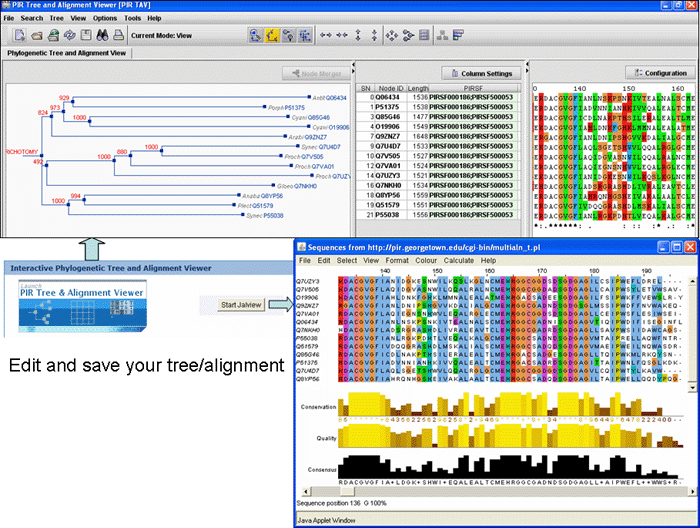
See sample output
FASTA Format
A sequence in FASTA format begins with a single-line description, followed by lines of sequence data. The description line is distinguished from the sequence data by a greater-than (">") symbol in the first column. One benefit of using FASTA format is that the sequence identifier will be reported with the results. An example sequence in FASTA format is:
>gi|3287971|sp|P37025|LIGT_ECOLI 2'-5' RNA ligase
MSEPQRLFFAIDLPAEIREQIIHWRATHFPPEAGRPVAADNLHLTLAFLGEVSAEK
EKALSLLAGRIRQPGFTLTLDDAGQWLRSRVVWLGMRQPPRGLIQLANMLRSQA
ARSGCFQSNRPFHPHITLLRDASEAVTIPPPGFNWSYAVTEFTLYASSFARGRTRY
TPLKRWALTQ
Pairwise Alignment Help
Insert two sequences using the
single letter
amino
acid code or enter two UniProtKB identifiers codes. Press the "Submit" button
and the alignment results will show the SSEARCH
Smith-Waterman full-length alignments between two
sequences (SSEARCH program version 3.4t24 July 21, 2004).
References
Smith TF, and MS Waterman (1981) Identification of common
molecular subsequences. J. Mol. Biol. 147,
195-197.
Pairwise alignment of P53039 and Q6FQ69
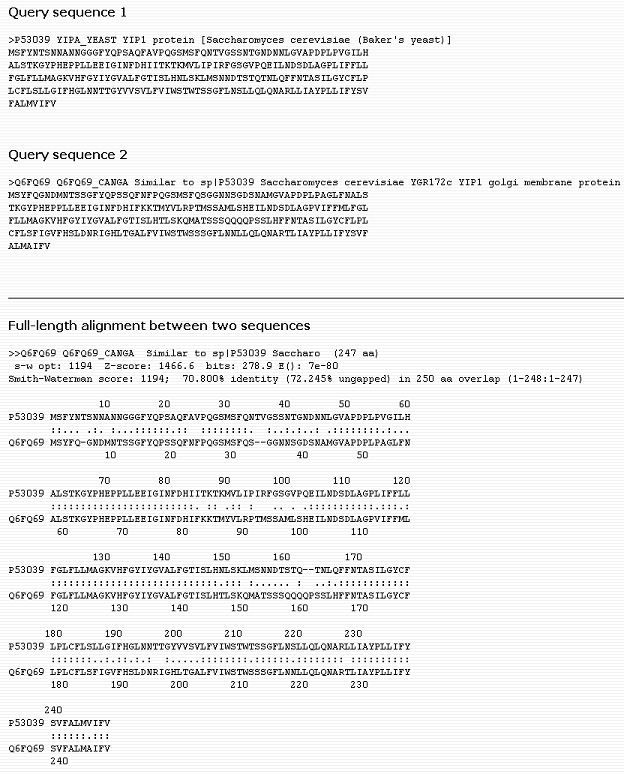
ID Mapping Help
This function allows the mapping of IDs between UniProtKB and other databases
First specify the type of ID of your source data (multiple types of ID can be selected by using the ctrl key). Specify the type of ID you want the mapping to. Type or paste the identifiers in the box provided separated by a space or return key. Alternatively, you can upload a file with a list of IDs. Select the output format and finally, press "Map".
The result page contains the list of ID matches. The IDs can be cut and pasted if needed or saved as a text file using the "save as" option provided by your web browser.
See annotated output below.
Mapping of GI numbers to UniProt ACs
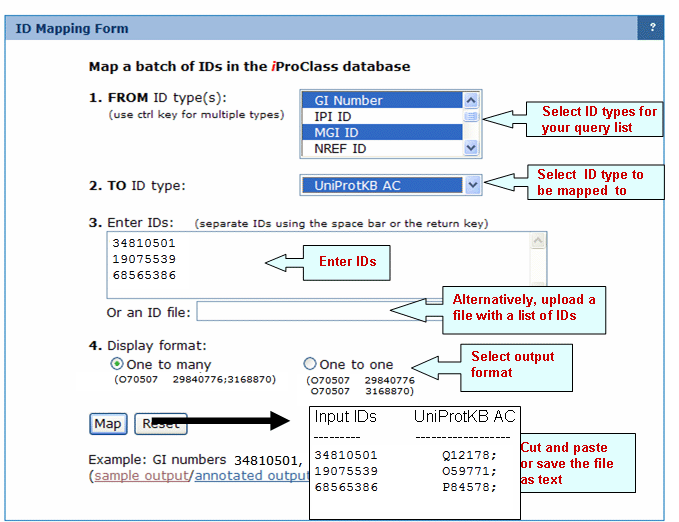
Composition/Molecular Weight Calculation
Help
Retrieve the composition
information and the molecular weight of a single or multiple sequences
based on the UniProtKB identifier or the single letter amino
acid code. The results contain the number of
residues and percentage for each amino acid in the
sequence. The calculation notes for the molecular
weight and the molecular weight for each amino
acid are also given.
Molecular weight composition for P53039

PIRSF
Scan
Search your
query sequence against the fully curated PIRSF
families. When you submit your query sequence, it
will be searched against the full-length and
domain HMM models for the fully curated PIRSFs by
HMMER program. If a match is found, the matched
regions and statistics will be displayed. The
query sequence should be entered using single
letter code or a UniProtKB identifier.
PIRSF scan for Q8Y5X7

Amino Acid Sequence Code Table
| Code |
Amino Acid |
| A | alanine |
| B | aspartate or asparagine |
| C | cysteine |
| D | aspartate |
| E | glutamate |
| F | phenylalanine |
| G | glycine |
| H | histidine |
| I | isoleucine |
| K | lysine |
| L | leucine |
| M | methionine |
| N | asparagine |
| O | pyrrolysine |
P | proline |
| Q | glutamine |
R | arginine |
| S | serine |
T | threonine |
| U | selenocysteine |
V | valine |
| W | tryptophan |
Y | tyrosine |
| Z | glutamic acid or glutamine | | BLAST and FASTA also accept |
| X | any |
| * | translation stop |
UniProtKB Identifiers for BLAST/FASTA
Search and Analysis Tools
The
following table shows examples for UniProtKB accession number and ID
|
| iProClass Report |
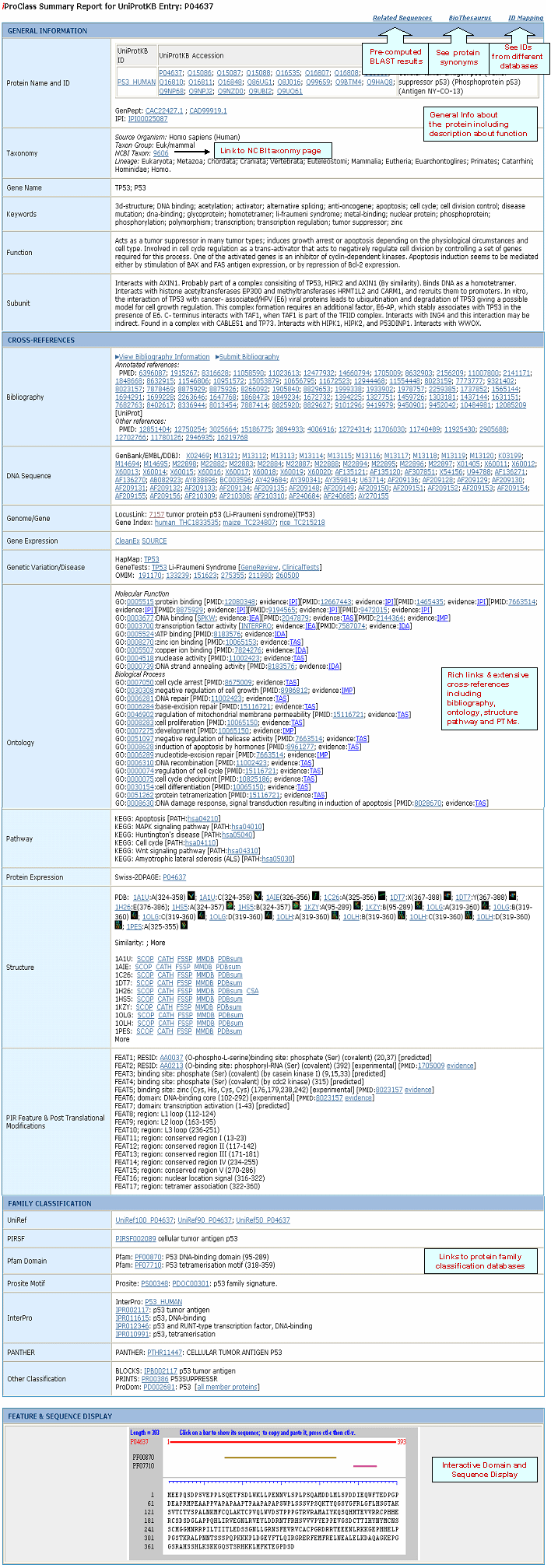 |
|
| PIRSF Report |
| Sample report for PIRSF000186. Information about the numbered fields is provided below. |
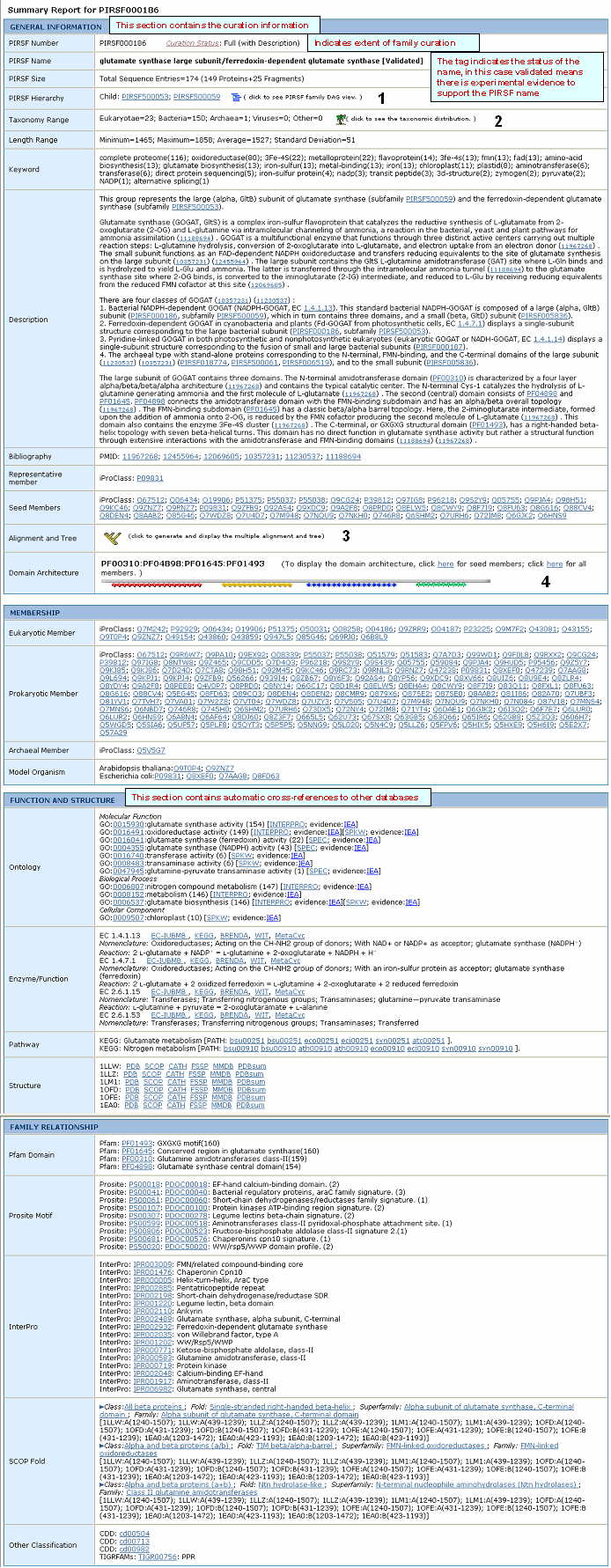 |
| 1- PIRSF Hierarchy DAG View |
 |
| 2- PIRSF Taxonomic Distribution |
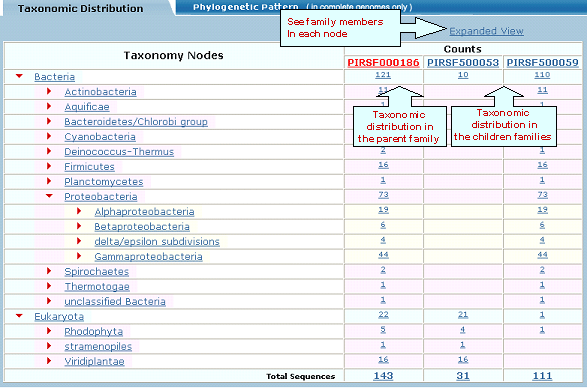 |
|
3- Multiple Alignment |
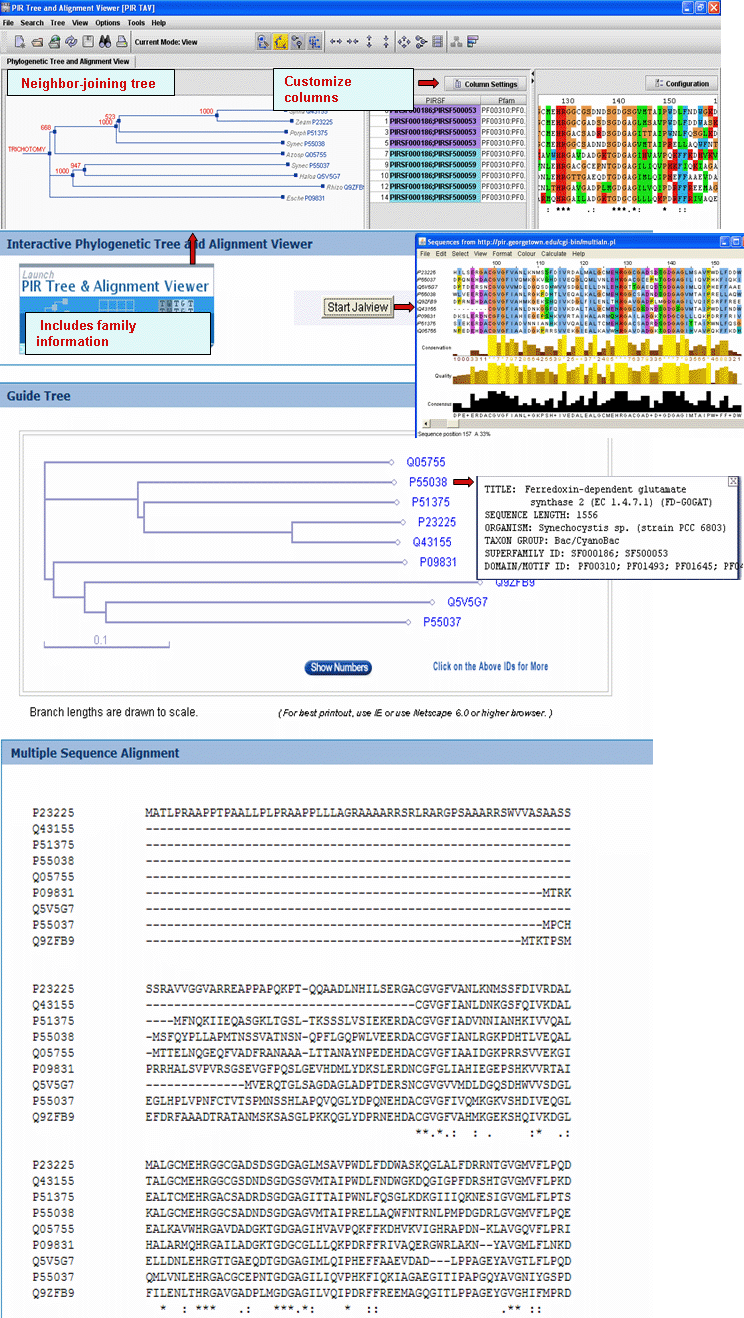 |
| 4- Domain Architecture |
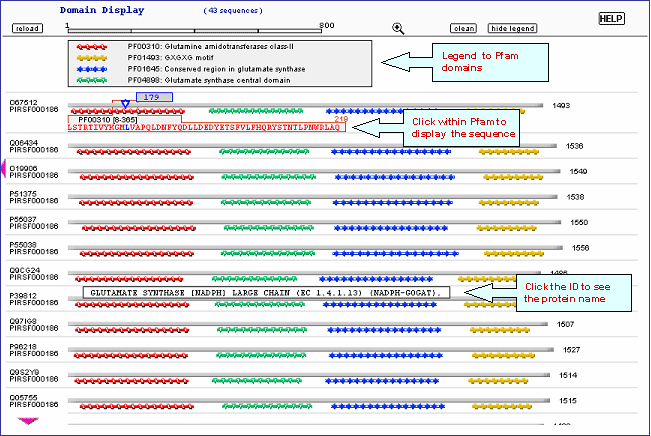 |
Master Protein Directory Help
This section describes the Master Protein Directory Text Search page. The Master Directory contains information on proteins and reagents identified by NIAID’s Biodefense Proteomics Research Centers. The overall layout of the page is similar to the regular PIR text search results however; it differs in the default columns displayed and has additional attributes to search. There are two columnar displays; 1) default display which is used to display multiple data types together (i.e. MS_proteomic and Microarray); and, 2) data type specific which is used when only one data type is selected and contains additional information that applies to that data type only. To view the data specific display from the pull-down menu --Select A Data Type to Show-- select a data type.
Default Display for Multiple Data Types
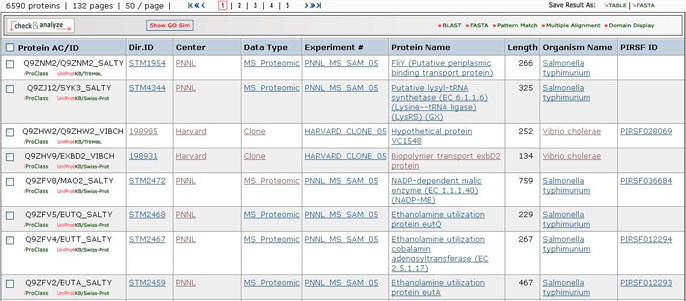
Data Type Specific Display (Microarray)
Search and Results Options
1- Browse Selection
By default the directory shows all data. This feature, a pull-down menu of options at the top of the page, allows you restrict your browsing by 1) data type, 2) Proteomics Research Center and 3) Organism
2- Search
Allows you to perform an additional search in case you want to further filter your output or you want to start a new search (no need to go back to the previous page). The available options are shown in a pull-down menu and are the same protein attributes as those found in the iProClass database with the addition of directory specific attributes listed under the – Master Directory – heading. Enter any text string or substring, case does not matter. If you wish to determine just the presence or absence of a value use the words “not null” or “null”. Wildcards are allowed.
3- Display Options
Depending on your specific need you can choose the columns to be displayed. To do this, click on the "Display Option" button, select the relevant field(s) in the "Fields Not in Display" list and transfer them to the "Fields in Display" list via the ">" button. Conversely, columns can be removed from display. Finally, click on "apply" for the changes to take effect.
4- Save Results As
The output can be saved to the user's local computer. The results will be saved for selected entries or, if no proteins are selected, for all entries. Clicking "Table" will save the displayed columns as a tab-delimited text file, which may be imported into a spreadsheet for easier viewing or analysis. Clicking "FASTA" will save the IDs and sequences in FASTA format.
Data Type Specific Display (Microarray)
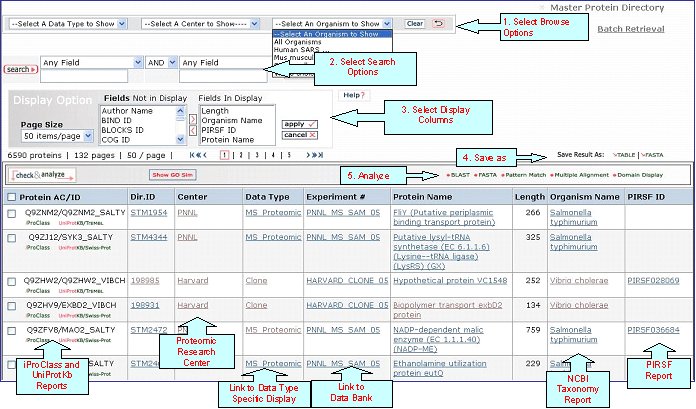
5- Analyze: BLAST, FASTA, Pattern
Match, Multiple Alignment and Domain
Display
Retrieved entries can be further
analyzed using the sequence analysis programs
available in the Results page. First, select the
protein(s) using the checkboxes on the left side
of the table, then click the corresponding
analysis tool.
Click "BLAST" or "FASTA" button, and a new
query page will be displayed, along with the
parameters that were selected in the initial
search.
Click "Pattern
Match" to search against the PROSITE database.
For multiple alignment, check at least 2
proteins (but no more than 50), then click the
"Multiple Alignment" button. A ClustalW
alignment and neighbor-joining tree will be
generated along with a link to the interactive PIR
viewer.
Domain display option, shows PFam domains (if
present) in graphical format.
6- Results Display
Results
of the search are displayed in a customizable
table. The exact columns displayed will depend on
the fields searched for, and user
preference.
Protein
AC/ID
The Protein AC/ID refers to the
UniProtKB or UniParc identifiers. Below these
numbers, you may choose either the iProClass
or the UniProtKB/UniParc
view of the protein report. The source of the
UniProtKB sequence is shown as
UniProtKB/Swiss-Prot or UniProtKB/TrEMBL if the
protein sequence is from Swiss-Prot or TrEMBL
section of UniProt Knowledgebase, respectively., respectively. Alternatively, the UniParc ID will be displayed if the sequence is not present in the UniProtKB database but is present in UniParc.
Dir.ID
The Directory ID is a unique identifier selected from the data submitted by the Proteomic Research Center. It is used in the master directory to identify a specific research result and to link protein information in the Master Directory to the detailed results in the Protein Bank.
Center
Identifies the NIAID Biodefense Proteomics Research Center that produced the data. Clicking the name show additional information on the center and data they submitted.
Data Type
Identifies the type of data provided. Clicking the Data type name opens a new window with protein information shown in the data type specific format.
Experiment #
Links to related experimental data in the protein data bank.
Protein Name
The common name given to a protein,
that identifies its function or specifies its
features.
Length
Number of amino acid
residues in the peptide or protein.
Organism Name
The genus and
species of the source organism from which the
sequence originated. Links to NCBI taxonomy
information are provided.
PIRSF ID
If a protein
belongs to a PIRSF family, then this column will
display the corresponding family identifier. Click
on the ID to retrieve the PIRSF report (see annotated
output).
7– Data Type Specific Search Options
All the common fields listed above can be searched as can any optional columns a user might add. In addition there are data type specific fields that are only displayed in the datatype specific display but can also be searched. Note: some reagent specific fields may be empty.
Searchable fields are:
AA Range – Used when data pertains to a specific domain only. Currently used for structures.
Comment – Project and protein specific annotation.
IEDB ID – Immune Eptitope ID, links to the Immune Epitope Database & Analysis Resource
Expression Condition – A controlled vocabulary currently used for Microarray and Mass Spectrometry Data. Selecting this option will provide a list of allowable terms. Though terms are shared between experiments not all terms exist in all experiments.
Expression Status – A controlled vocabulary currently used for Microarray and Mass Spectrometry Data. Selecting this option will provide a list of allowable terms. Though terms are shared between experiments not all terms exist in all experiments.
GO Slim Func. – GO slim biological functional terms associated with the protein.
GO Slim Comp - GO slim biological compartment terms associated with the protein.
GO Slim Proc. - GO slim biological process terms associated with the protein.
Insertion Point – Used for clones to indicate the insertion site on the vector.
Interaction ID – Unique ID for interaction.
Clone ID – Unique ID for the clones insert.
PDB ID – Protein Data Bank ID for protein structures.
Prey AC – iProclass Accession for prey protein in a Y2H interacting pair
Publication – PRC publication related to the protein.
Vector – Name of cloning Vector
BRC Link – Bioinformatics Resource Center link. Protein specific link to one of the NIAID funded BRC centers database.
Complete Predicted Proteomes Help
This tool allows text searches restricted to complete proteomes as annotated by the UniProt Consortium and being studied by the Biodefense Proteomics Research Program. The results are linked to the Master Protein Directory which contains experimental information about some of the proteins.
To begin select the proteomes of interest and search.
Search and Results Options
1- Search
Once in the tool you can further restrict your search. The available options are shown in a pull-down menu and are the same protein attributes as those found in the iProClass database with the addition of the - Master Directory – (MD) option . Enter any text string or substring, case does not matter. If you wish to determine just the presence (+) or absence (blank) of a value use the words “not null” or “null”. Wildcard searches are allowed.
2- Display Options
Depending on your specific need you can choose the columns to be displayed. To do this, click on the "Display Option" button, select the relevant field(s) in the "Fields Not in Display" list and transfer them to the "Fields in Display" list via the ">" button. Conversely, columns can be removed from display. Finally, click on "apply" for the changes to take effect.
3- Save Results As
The output can be saved to the user's local computer. The results will be saved for selected entries or, if no proteins are selected, for all entries. Clicking "Table" will save the displayed columns as a tab-delimited text file, which may be imported into a spreadsheet for easier viewing or analysis. Clicking "FASTA" will save the IDs and sequences in FASTA format.
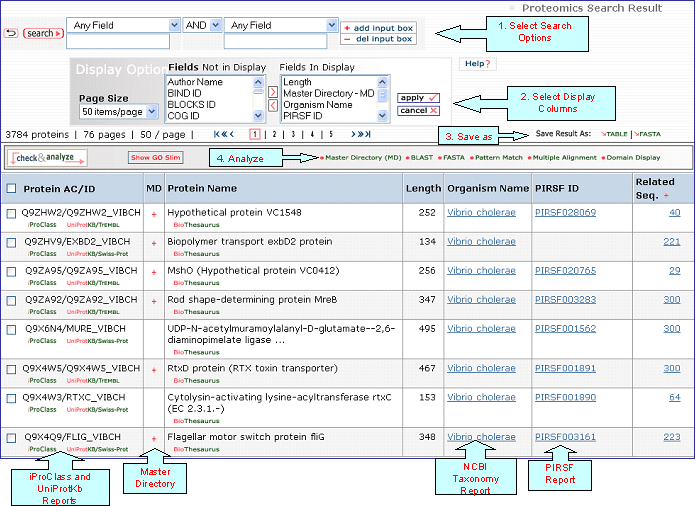
4- Analyze: Master Directory, BLAST, FASTA, Pattern
Match, Multiple Alignment and Domain
Display
Retrieved entries can be further analyzed using the Master Directory or using the sequence analysis programs available in on the PIR website. Master Directory: Any protein with information present in the Master Directory of the Biodefense Proteomics Research Program is indicated with a (+) in the MD column. Checking these entries and then selecting ‘Master Directory’ in the analyze tool bar opens the Master Directory with the selected proteins shown. Sequence Analysis: Selecting these options sends you to the PIR website where the analysis is performed. To return to the Master Directory use your browser back button. First, select the protein(s) using the checkboxes on the left side of the table, then click the corresponding analysis tool.
Click "BLAST" or "FASTA" button, and a new
query page will be displayed, along with the
parameters that were selected in the initial
search.
Click "Pattern
Match" to search against the PROSITE database.
For multiple alignment, check at least 2
proteins (but no more than 50), then click the
"Multiple Alignment" button. A ClustalW
alignment and neighbor-joining tree will be
generated along with a link to the interactive PIR
viewer.
Domain display option, shows PFam domains (if
present) in graphical format.
5- Results Display
Results
of the search are displayed in a customizable
table. The exact columns displayed will depend on
the fields searched for, and user
preference.
Protein AC/ID
The Protein AC/ID refers to the UniProtKB or UniParc identifiers. Below these numbers, you may choose either the iProClass or the UniProtKB/UniParc view of the protein report. The source of the UniProtKB sequence is shown as UniProtKB/Swiss-Prot or UniProtKB/TrEMBL if the protein sequence is from Swiss-Prot or TrEMBL section, respectively. Alternatively, the UniParc ID will be displayed if the sequence is no present in the UniProtKB database along with the UniParc report.
MD (Master Directory)
Any protein with information present in the Master Directory of the Biodefense Proteomics Research Program is indicated with a (+) in the MD column. To restrict your search to only proteins that are present in the MD select “Master Directory” in the search field options and enter the value “yes” or “not null”.
Protein
Name
The common name given to a protein,
that identifies its function or specifies its
features.
Length
Number of amino acid
residues in the peptide or protein.
Organism Name
The genus and
species of the source organism from which the
sequence originated. Links to NCBI taxonomy
information are provided.
PIRSF ID
If a protein
belongs to a PIRSF family, then this column will
display the corresponding family identifier. Click
on the ID to retrieve the PIRSF report (see annotated
output).
Related
Seq.
This column shows the number of
pre-computed BLAST hits obtained using default
parameters. Only up to 300 sequences will be
displayed. By clicking the number you access to
the related sequence page. This allows to have a
glance at sequence similarity in a very fast way.
The number itself already provides some
information about how unique the protein is. For
example, a very low number may tell you that the
query is specific to a certain species, genus,
taxon, etc. The "+" sign next to the Related
Sequence title allows to compare number of related
sequences at 3 different E-value cut-offs as shown
below.

GO Slim Analysis
GO slims are smaller versions of the Gene Ontologies containing a subset of the terms in the whole GO. They give a broad overview of the ontology content without the detail of the specific fine grained terms. GO slims are particularly useful for giving a summary of the results of GO annotation of a genome or proteome when broad classification of gene product function is required.
You can view the GO slim terms for Biological Function, Component, and Process by selecting the “Show GO Slim” button in the analysis tool bar. You can then view statistics for the individual ontologies (Function, Component, and Function) by checking entries of interest and selecting the ontology to show. Follow the GO ID links to learn more about the GO term.
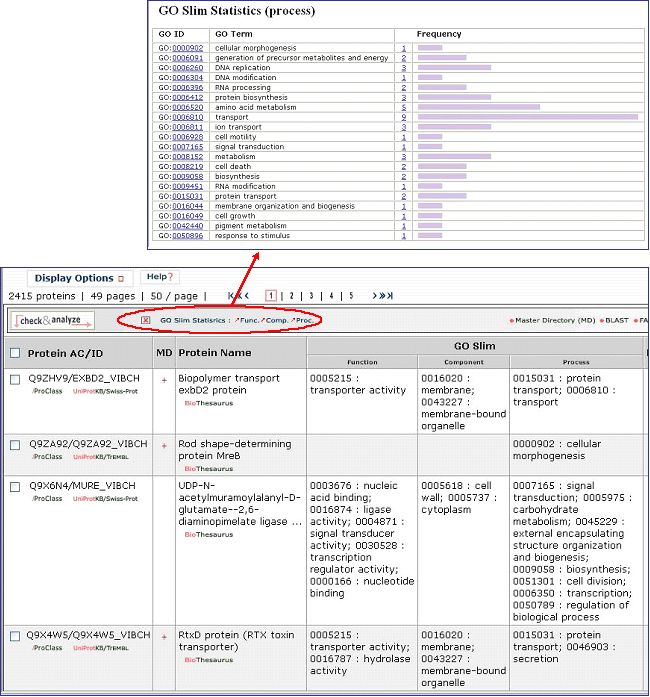
Master Reagent Directory Help
This section describes the Master Reagent Directory Text Search page. The Reagent Directory contains reagents identified by NIAID’s Biodefense Proteomics Research Centers and provides links to repositories were reagents can be obtained. The overall layout of the page is similar to the regular PIR text search results however; it differs in the default columns displayed and has additional attributes to search. The are two columnar displays; 1) default display which is used to display multiple reagent types together (i.e. antibodies and clones); and, 2) reagent type specific which is used when only one reagent type is selected and contains additional information that applies to that reagent only.
Default Display for Multiple Reagent Types

Reagent Type Specific Display (Antibody)
Search and Results Options
1- Browse Selection
By default the directory shows all reagents. This feature, a pull-down menu of options, allows you restrict your browsing by 1) Reagent type, 2) Proteomics Research Center and 3) Organism
2- Search
Allows you to perform an additional search in case you want to further filter your output or you want to start a new search (no need to go back to the previous page). The available options are shown in a pull-down menu (see current options below). Enter any text string or substring, case does not matter. If you wish to determine just the presence or absence of a value use the words “not null” or “null”.
3- Display Options
Depending on your specific need you can choose the columns to be displayed. To do this, click on the "Display Option" button, select the relevant field(s) in the "Fields Not in Display" list and transfer them to the "Fields in Display" list via the ">" button. Conversely, columns can be removed from display. Finally, click on "apply" for the changes to take effect.
4- Save Results As
The output can be saved to the user's local computer. The results will be saved for selected entries or, if no reagents are selected, for all entries. Clicking "Table" will save the displayed columns as a tab-delimited text file, which may be imported into a spreadsheet for easier viewing or analysis.

5- Results Display
Results of the search are displayed in a customizable table. The exact columns displayed will depend on the fields searched for, and user preference. The default columns are described below.
Reagent ID
The Reagent ID refers is a unique identifier. If the Reagent is deposited in the BEI resource is the same ID used at BEI.
Reagent Type
Identifies the type of reagent. Clicking the reagent type name opens a new window with reagent information shown in the reagent specific format.
Name/Description
Descriptive text about the reagent.
Organism Name
The genus and species name of the source organism from which or for which the reagent was developed. For example: Bacterial strains list the bacterial species name here, not the strain. Antibodies though originating from mouse or rabbit list the antigens organism. Links to NCBI taxonomy information are provided.
Center
Identifies the NIAID Biodefense Proteomics Research Center that produced the reagent. Clicking the name show additional information on the center and data they submitted.
Experiment #
Links to related experimental data in the protein data bank. If these reagents were used for a particular set of experiments this number would link to that data.
Publication
Links to PRC publications that used the reagent.
Source
Links to reagent repositories where users can obtain the reagent.
6- Reagent Specific Search Options
All the common fields listed above can be searched. In addition there are reagent specific fields that are only displayed in the reagent specific display but can also be searched. Note: some reagent specific fields may be empty.
Searchable fields are:
Taxonomy ID – NCBI Taxonomy ID.
Ab EBPC – Internal tracking number for Einstein Biodefense Proteomics Center
Ab ELISA Data – Elisa titers
Ab EM? – Value = Y, N, null. Y means spot detected in Electron Microscopy.
Ab IFA? - Value = Y, N, null. Y means spot detected in Immunofluorescence Assay.
Ab Type – Value = Monoclonal, Polyclonal, null
Ab Western? - Y, N, null. Y means band detected in Western blot.
Ab Peptide – Peptides used to immunize and for Eliza
Bac Parent ID – Parent Strain Identifier
Bac Strain ID – Reagent Strain ID
Clone Insertion Point – Insertion point on clone vector.
Clone Locus ID – Locus ID of insert sequence.
Clone Vector ID – Name of Vector used
Clone Protein ID – iProclass accession or protein, links to Master Protein Directory.
Reference Proteomes Help
1. What are Reference Proteomes?
Reference Proteomes (RPs), are proteomes that are selected from Representative Proteome Groups (RPGs) containing similar proteomes calculated based on co-membership in UniRef50 clusters. Reference Proteome is the proteome that can best represent all the proteomes in its group in terms of the majority of the sequence space and information. RPs at 75%, 55%, 35% and 15% co-membership threshold are provided to allow users to decrease or increase the granularity of the sequence space based on their requirements.
2. Reference Proteomes BLAST search.
1. Select a Database
BLAST search can be performed against RP75, RP55, RP35 or RP15:
Lower RPs (such as RP15) has fewer proteomes than higher RPs (such as RP75). For example, to get the least number of BLAST results choose RP15. For additional help on BLAST please see https://proteininformationresource.org/pirwww/support/help.shtml#3.
3. Availability and usage of RP.
For the threshold values of 75, 55, 35, and 15 (RP75, RP55, RP35 and RP15), corresponding Representative Proteome Group files are provided, via links from the RP home page, in the format as below:
>rp_taxon_id rp_code rp_name rp_annotation_score (AS) taxon_group C(THRESHOLD)
mp_taxon_id mp_code mp_name mp_annotation_score (AS) taxon_group X_to_rp(X)
...
>rp_taxon_id rp_code rp_name rp_annotation_score (AS) taxon_group C(THRESHOLD)
...
Where
rp is Reference Proteome,
mp is Member Proteome in the Representative Proteome Group
An example of Representative Proteome Group is shown below:
>205920 EHRCR Ehrlichia chaffeensis (strain Arkansas) Bac/Alpha-proteo 1111.19332(AS) 55(CUTOFF)
269484 EHRCJ Ehrlichia canis (strain Jake) Bac/Alpha-proteo 1111.10824(AS) 71.20366(X)
302409 EHRRG Ehrlichia ruminantium (strain Gardel) Bac/Alpha-proteo 1111.10730(AS) 64.05622(X)
254945 EHRRW Ehrlichia ruminantium (strain Welgevonden) Bac/Alpha-proteo 1111.12521(AS) 65.56531(X)
Also provided are the sequence files in FASTA format for the RP75, RP55, RP35 and RP15 sets. Users can choose to make their own customized RP set by using the taxon-based table or perl script available via a link from the RP home page. For example, we suspect that, for some users, the ideal set could be RP75 for Animals + RP55 for other cellular organisms + any missing GO Reference Genomes.
4. Reference Proteome availability from the iProClass interface.
iProClass is an integrated data-warehouse containing all UniProtKB proteins and additional proteins from NCBI resources. The proteins from the defined representative proteome sets are indexed in iProClass and are available for BLAST (http://proteininformationresource.org/rps/blast_rp.shtml). Additionally, all the proteins from the RP55 set can be retrieved from http://proteininformationresource.org/pirwww/search/textsearch.shtml by selecting Rep Proteome and then typing in not null and clicking on Search. Users can perform additional filtering on the retrieved set by performing Boolean searches using more than 65 fields available from the text search pull down menu. The BLAST and the text search results can be downloaded from the results page for further analysis.
5. Browsing the Reference Proteomes.
The RPs at the four different thresholds can be viewed at http://proteininformationresource.org/cgi-bin/rps_tree.pl. The top most nodes are Archaea, Bacteria and Eukaryota and the fully expanded view shows all the proteomes that have been analyzed to identify the RPs. Browsing the RPs at different threshold for different taxonomy nodes can provide clues as to which CMT is best for a particular branch and how the RPs are distributed in the taxonomy tree. Once a desired set of RPs is displayed on the screen, it can be printed for future reference.
6. Reference Proteomes data update.
The protein sets used for text search and BLAST search are updated every four weeks. New proteomes are added every six months. All releases are archived for at least 5 years.
|
|


